��Ŀ����
����Ŀ������ӵ�����ִ������ܵ�صĴ����������ܵĵ缫���������ʽṹ������ء�
(l) LiFePO4��������õĽṹ�ȶ��Զ���Ϊ��һ���������ϣ�����PO43-�Ľṹ������أ�PO43-�����幹��Ϊ____��P��O��S�ĵ縺�ԴӴ�С��˳��Ϊ______________
(2)ͨ���ڵ缫���ϱ��������̼��������������ǿ�䵼���ԡ�����Ѫ�� ��������̼������̼Դ����������ˮ��ԭ���� ___������Ѫ����̼ԭ�ӵ��ӻ���ʽΪ ___��1mol����Ѫ��������̼ԭ�ӵ���ĿΪ______
��������̼������̼Դ����������ˮ��ԭ���� ___������Ѫ����̼ԭ�ӵ��ӻ���ʽΪ ___��1mol����Ѫ��������̼ԭ�ӵ���ĿΪ______
(3) Li+�����ѳ�����﮵�ؽṹ̮������O2����ը��ʵ��֤ʵO2����е����Ӷ���Ϊ˳���Է��ӣ����нṹʽ���ڵ�������ӣ������п��ܴ���O2���ӽṹ����____�����ţ���
A. ![]()
B. 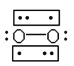
C. ![]()
D. 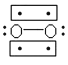
(4)Ŀǰ��õĵ缫��������ܸ����������ʯī��
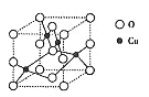
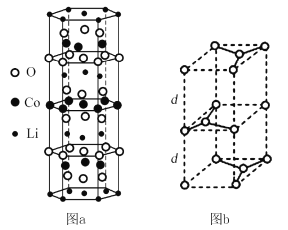
����ܸ�����������Li��Co��O�ֱ��γ������߲�״�ṹ��ͼa��������Li-O-Co-O-Li�CO-Co-O- Li--˳�����У���û�����Ļ�ѧʽΪ____��Co3+�ļ۲�����Ų�ʽΪ_____��
��ʯī������ͼb������Ϊd pm��C��C����Ϊa pm��ʯī������ܶ�Ϊp g/cm3����ʽ��ʾ�����ӵ�����Ϊ____mol-l��
���𰸡��������� O>S>P �����к��ж���ǻ�������ˮ���Ӽ��γ���� sp2��sp3 2NA B LiCoO2 3d6 ![]()
��������
(l) ���ݼ۲���ӶԻ������ۣ�������������ӵļ۵��Ӷ������ҳ�PO43-�ռ乹�ͣ�P��O��S�ĵ縺�ԴӴ�С��˳�ǽ�����ǿ���͵縺�Դ�С֮��Ĺ�ϵ���ش�
(2)����Ѫ��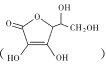 ������ˮ��ԭ���Ӱ���ܽ��Ե����ء������ǻ�������ˮ������������������Ѫ����̼ԭ�ӵ��ӻ���ʽ��̼̼���Ƕȷ�����1mol����Ѫ��������̼ԭ�ӵ���Ŀ��϶����ͼʾ�ṹ�жϣ�
������ˮ��ԭ���Ӱ���ܽ��Ե����ء������ǻ�������ˮ������������������Ѫ����̼ԭ�ӵ��ӻ���ʽ��̼̼���Ƕȷ�����1mol����Ѫ��������̼ԭ�ӵ���Ŀ��϶����ͼʾ�ṹ�жϣ�
(3)�����Ϣ��ʾ��ͼѡ���жϣ�
(4)��Ϣ���ܸ�����������Li��Co��O�ֱ��γ������߲�״�ṹ��ͼa�����þ�̯�����㾧���ڵ�ԭ��������û�����Ļ�ѧʽ���ܵ�ԭ������Ϊ27�������Ų����ɿ�дCo3+�ļ۲�����Ų�ʽ��
����֪ʯī������ͼb������Ϊd pm��C��C����Ϊa pm�����������ܶȵ��ھ�����ܶ�Ϊp g/cm3����ʽ���ӵ�������
(l) PO43-��Pԭ�Ӽ۲���ӶԸ���![]() �Ҳ����µ��Ӷԣ����ݼ۲���ӶԻ��������жϿռ乹��Ϊ��������ṹ��
�Ҳ����µ��Ӷԣ����ݼ۲���ӶԻ��������жϿռ乹��Ϊ��������ṹ��
��Ϊ���������壻
Ԫ�صķǽ�����Խǿ����縺��Խ����縺��O>S>P��
����O>S>P��
(2)����Ѫ�� ̼ԭ����Ŀ��Խ��٣��������ں�4���ǻ�������ˮ���Ӽ��γ�����������������ˮ��
̼ԭ����Ŀ��Խ��٣��������ں�4���ǻ�������ˮ���Ӽ��γ�����������������ˮ��
��Ϊ�������к��ж���ǻ�������ˮ���Ӽ��γ������
��������̼̼��������Ӧ̼ԭ��sp3�ӻ���ʽ��Ҳ��̼̼˫������Ӧ̼ԭ��sp2�ӻ���ʽ��
����sp2��sp3��
���ĸ���ͬ��ԭ�ӻ�ԭ����������̼ԭ�ӳ�Ϊ����̼ԭ�ӣ���ͼ֪��1������Ѫ������к�2������̼ԭ�ӣ���1mol����Ѫ��������̼ԭ�ӵ���ĿΪ2NA��
����2NA��
(3)�����Ϣ��O2����е����Ӷ���Ϊ˳���Է��ӣ���ʾ��ͼ֪B�ṹ�л�ѧ����3���ӣ�B�е����ӣ�B���㣻
����B��
(4) ��ܸ�����������Li��Co��O�ֱ��γ������߲�״�ṹ��ͼa���������������ṹ�У�Li����Ϊ��![]() ����Co����Ϊ��
����Co������![]() ��O������
��O����Ϊ��![]() ��Li��Co��O������Ϊ1��1��2����ѧʽΪLiCoO2��
��Li��Co��O������Ϊ1��1��2����ѧʽΪLiCoO2��
����LiCoO2��
�ܵ�ԭ������Ϊ27��Co3+��24�����ӣ������Ų�ʽΪ[Ar] 3d6����۲�����Ų�ʽΪ3d6��
����3d6��
ʯī������̼ԭ����Ŀ=![]() ���ʾ���������=
���ʾ���������=![]() �����ڣ�ƽ���ı��Σ�C��C����Ϊa pm=
�����ڣ�ƽ���ı��Σ�C��C����Ϊa pm=![]() ������ı߳�Ϊ
������ı߳�Ϊ![]() �������ϵĸ�Ϊ
�������ϵĸ�Ϊ![]() ����ı߳�=
����ı߳�=![]() ������Ϊd pm���������=
������Ϊd pm���������=![]() ��
��![]() ��
��![]() =
=![]() ����þ������ܶ�
����þ������ܶ� ����NA=
����NA=![]() ��
��
���� ![]() ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�����ſƼ��Ľ���������������Դ������������Ϊ��������ע�Ľ��㡣�װ�Ǧ��(CH3NH3PbI3)����ȫ��̬���ѿ�����̫���ܵ�ص�������������CH3NH2��PbI2��HIΪԭ�Ϻϳɣ��ش��������⣺
��1����ȡ�װ��ķ�ӦΪCH3OH(g)��NH3(g) ![]() CH3NH2(g)��H2O(g) ��H����֪�÷�Ӧ����ػ�ѧ���ļ����������£�
CH3NH2(g)��H2O(g) ��H����֪�÷�Ӧ����ػ�ѧ���ļ����������£�
���ۼ� | C-O | H-O | N-H | C-N |
����/kJ��mol��1 | 351 | 463 | 393 | 293 |
��÷�Ӧ����H��_______��
��2��������Ӧ������ļ״���ҵ������ˮú���ϳɣ���ӦΪCO(g)��2H2(g) ![]() CH3OH(g) ��H��0����һ�������£���1 mol CO��2 mol H2ͨ���ܱ������н��з�Ӧ�����ı�ijһ�������(�¶Ȼ�ѹǿ)ʱ��CH3OH�����������(CH3OH)�仯������ͼ��ʾ��
CH3OH(g) ��H��0����һ�������£���1 mol CO��2 mol H2ͨ���ܱ������н��з�Ӧ�����ı�ijһ�������(�¶Ȼ�ѹǿ)ʱ��CH3OH�����������(CH3OH)�仯������ͼ��ʾ��

��ƽ��ʱ��M��CH3OH���������Ϊ10%����CO��ת����Ϊ________��
��X����a�����ֵ��b��________(���С��)��ijͬѧ��Ϊ��ͼ��Y���ʾ�¶ȣ�����Ϊ���жϵ�������_____________________��
��3��ʵ���ҿ�����������Ǧ������ᷴӦ�Ʊ����ܵ�PbI2����ÿ����3 mol PbI2�ķ�Ӧ�У�ת�Ƶ��ӵ����ʵ���Ϊ__________��
��4�������£�PbI2������Һ(�ʻ�ɫ)��c(Pb2��)=1.0��10��3mol��L��1����Ksp(PbI2)��_________����֪Ksp(PbCl2)��1.6��10��5����ת����ӦPbCl2(s)��2I-(aq) ![]() PbI2(s)��2Cl-(aq)��ƽ�ⳣ��K��_________��
PbI2(s)��2Cl-(aq)��ƽ�ⳣ��K��_________��
��5���ֽ�HI���ߺ�Һ�෨�Ʊ�HI��Ӧ���߷ֱ���ͼ1��ͼ2��ʾ��

�ٷ�ӦH2(g)��I2(g) =2HI(g) ����H__________(����ڻ�С��)0��
�ڽ���������ͨ���ˮ�лᷢ����Ӧ��SO2��I2��2H2O=3H����HSO4����2I����I2��I��![]() I3����ͼ2������a��b�ֱ����������________��_________���������ţ�����ͼ2 ֪Ҫ��ߵ�Ļ�ԭ�ʣ��������¶��⣬�����Բ�ȡ�Ĵ�ʩ��___________________________________��
I3����ͼ2������a��b�ֱ����������________��_________���������ţ�����ͼ2 ֪Ҫ��ߵ�Ļ�ԭ�ʣ��������¶��⣬�����Բ�ȡ�Ĵ�ʩ��___________________________________��