��Ŀ����
8��ij��ѧ��ȤС��Ϊ�˲ⶨ��̼�Ͻ�����̼���ֵ��ʵĻ������������������������ͼ��ʾ��ʵ��װ�ã��г�������ʡ�ԣ���ʵ�鷽������ʵ��̽����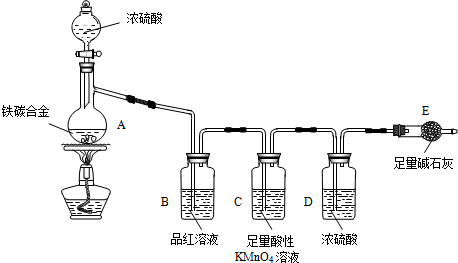
��1���������װ�������Ե�һ�ַ����ǣ��رշ�Һ©���Ļ�������Eװ�ú�������һ�����ܣ�Ȼ��ѵ��ܷ���ʢ��ˮ��ˮ���У�����ƿ��������ܿ������ݲ�����ֹͣ���Ⱥܲ���һ��ˮ������֤��װ�õ����������ã�
��2������E������������a g��̼�Ͻ���Ʒ����װ��A�У��ټ���������Ũ���ᣬ���ȣ���A�в����ݳ�����ʱ��ֹͣ���ȣ�����E�����أ�E����b g����̼�Ͻ���������������Ϊ$\frac{11m-3b}{11m}$��100%��д����ʽ����
��3��װ��C�����ó�ȥCO2�е�SO2��
s5
��4����ͬѧ��Ϊ�����ݴ�ʵ���õ����ݣ�����Ͻ������������������ܻ�ƫ�ͣ�ԭ���ǿ�����CO2��ˮ��������E��ʹb��������Ϊ�Ľ��ķ�����Eװ�ú�������һ��ʢ��ʯ�ҵĸ���ܣ�
��5����ͬѧ��Ϊ����ʹ��ͬѧ��Ϊ��ƫ��õ��Ľ������ݴ�ʵ���úϽ���������������Ҳ���ܻ�ƫ�ߣ�����Ϊ���е�ԭ���Ƿ�Ӧ������CO2����δ����ȫ�ŵ�װ��E�У�����bƫ�ͣ�
���� ��1������װ���е�ѹǿ�仯����Һ��仯�������飬������ܲ���ˮ�����ȷ���װ�õ�����ð���ݣ�ֹͣ��������һ��ˮ����
��2������mg��̼�Ͻ𣬼������Ũ���ᣬE����bg�������ɶ�����̼������Ϊbg�����������غ㶨�ɣ�������mg��̼�Ͻ��к�̼Ԫ�ص��������������������������
��3��װ��C�����ø��������Һ��ǿ���������ն�������
��4��Eװ�ú�������һ��ʢ��ʯ�ҵĸ���ܷ�ֹ�����еĶ�����̼��ˮ�����������ܣ�
��5��װ�������ɵĶ�����̼����ȫ������ʯ�����գ�
��� �⣺��1���رշ�Һ©���Ļ�������Eװ�ú�������һ�����ܣ�Ȼ���������ܲ���ˮ�����ȷ���װ�õ�����ð���ݣ�ֹͣ��������һ��ˮ��֤��װ�������Ժã�
�ʴ�Ϊ���������ܲ���ˮ�У�������ƿ��������ð���ݣ�ֹͣ������һ��ˮ��������
��2����ȡmg��̼�Ͻ𣬼������Ũ���ᣬ���ȴ�A�в����ݳ�����ʱ��ֹͣ���ȣ�����Eװ�ò����أ�E����bg�������ɶ�����̼������Ϊbg�����������غ㶨�ɣ���mg��̼�Ͻ��к�̼Ԫ�ص�����Ϊ$\frac{12b}{44}$g������������Ϊmg-$\frac{3b}{11}$g��������������Ϊ$\frac{11m-3b}{11m}$��100%��
�ʴ�Ϊ��$\frac{11m-3b}{11m}$��100%��
��3��װ��C�����ø��������Һ��ǿ���������ն�������5SO2+2KMnO4+2H2O=K2SO4+2MnSO4+2H2SO4 ��
�ʴ�Ϊ����ȥCO2�е�SO2��
��4��E����װ�Լ�Ϊ��ʯ�ң������տ�����CO2��H2Oʹb���Ľ��ķ���������һ��ʢ��ʯ�ҵĸ���ܷ�ֹ�����еĶ�����̼��ˮ���룻
�ʴ�Ϊ��Eװ�ú�������һ��ʢ��ʯ�ҵĸ���ܣ�
��5��װ�������ɵĶ�����̼����ȫ������ʯ�����ջᵼ�²ⶨ���ƫ�ͣ��ʴ�Ϊ����Ӧ������CO2����δ����ȫ�ŵ�װ��E�У�����bƫ�ͣ�
���� ���⿼�����������ʵ�̽��ʵ�鷽����װ�õ��������������̷�Ӧ���Լ������ǽ���ؼ�����Ŀ�ѶȽϴ�

 �������ϵ�д�
�������ϵ�д�| A�� | ���� | B�� | ��ͪ | C�� | ��ȩ | D�� | �������� |
| A�� | �ϳ��� | B�� | ���� | C�� | �ϳ���ά | D�� | �մ� |
| A�� | ���� | B�� | ���� | C�� | ��ȡ | D�� | �������� |
| A�� | Ԫ��W��X���Ȼ����У���ԭ�Ӿ�����8���ӵ��ȶ��ṹ | |
| B�� | Ԫ��X��һ�ֵ�������Ȼ����Ӳ���������� | |
| C�� | Ԫ��Y�ĵ���ֻ����������ᷴӦ���������������ܺͼӦ | |
| D�� | Ԫ��Z����Ԫ��X�γɹ��ۻ�����XZ2 |
��1������[CO��NH2��2]������泥�NH4CNO����Ϊͬ���칹�壻������������ӻ����ѡ������ӡ����ۡ�����
��2��Һ����һ��������ϣ���̬��ת��ΪҺ�����ͷ�������ѡ������ա����ͷš�����Һ����ͨ��ͼ1װ���ͷ��������ù���������ת����ʽΪ����ת��Ϊ��ѧ�ܣ�
��3����������Ϊ���������ں��º����ܱ������г���һ������NO��NH3����һ�������·�����Ӧ��6NO��g��+4NH3��g��?5N2��g��+6H2O��g����
����˵���÷�Ӧ�Ѵﵽƽ��״̬�ı�־��bc��������ѡ��
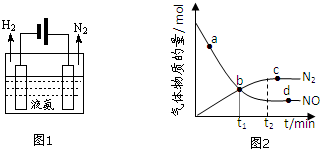
a����Ӧ���ʦͣ� NH3��=�ͣ� N2��
b��������ѹǿ������ʱ��������仯
c��������N2�����ʵ�������������ʱ��������仯
d��������n��NO����n��NH3����n��N2����n��H2O��=6��4��5��6
��ij��ʵ���в��������NO��N2�����ʵ�����ʱ��仯��ͼ2��ʾ��ͼ�Цͣ�������ͣ��棩��ȵĵ�Ϊcd��ѡ����ĸ����
��4����֪���Ͽ�1mol���ۼ����յ��������γ�1mol���ۼ��ͷŵ������������
| ���ۼ� | H-H | N-H | N��N |
| �����仯/kJ•mol-1 | 436 | 390.8 | 946 |
��5����ҵ���ð�ˮ�������Ṥҵβ���е�SO2���ȿ�������Ⱦ�ֿɻ��NH4HSO3�Ȳ�Ʒ������1000kg ��NH3��������Ϊ17%�İ�ˮ����SO2��ȫ��ת��ΪNH4HSO3�������������ɱ�����ɻ�õ�����Ϊ782Ԫ����������ļ۸������
| NH3��������17%�İ�ˮ | ��ˮNH4HSO3 | |
| �۸�Ԫ/kg�� | 1.0 | 1.8 |
| A�� | ����ͨ��������ǹ��� | B�� | ��Ļ�ԭ�Ա����� | ||
| C�� | As2O5��Ӧˮ��������Ա�H3PO4�� | D�� | ���Դ���-3��+5�ȶ��ֻ��ϼ� |
| A�� | FeO��HNO3 | B�� | Al��OH��3��HNO3 | C�� | H2S��HNO3 | D�� | NH3��HNO3 |

| A�� | 1 mol��������������ˮ��Ӧ����6 mol Br2 | |
| B�� | 1 mol�����ʿ���5 molNaOH��Ӧ | |
| C�� | ����������ԭ�ӿ�����ͬһƽ���� | |
| D�� | ����ʹ����KMnO4��Һ����ɫ��ȥ�����֤�������ʷ����к���̼̼˫�� |