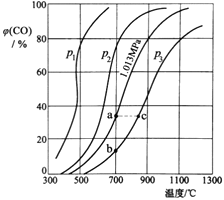
��Ŀ����
����Ŀ�������Ƶķ������ܻ�ó�������ۣ�����Ƴ��Ľ�����Ҫ����ʵ���ļ������ȷ������ȷ��������й�������ȷ���ǣ� ��
��BrCl�Ļ�ѧ���ʺ�Cl2���ƣ�Cl2��ˮ��Ӧ��������ԭ��Ӧ��BrCl+H2O=HCl+HBrOҲ��������ԭ��Ӧ
��Fe3O4����д��FeOFe2O3����ʽ��Pb3O4Ҳ��д��PbOPb2O3����ʽ
��37��ʱ��Fe3+�ܴ�H2O2�ķֽ⣻80��ʱ��MnO2����������øҲ�ܴ�H2O2�ķֽ�
�ܵ��CuCl2��Һ��������Cu�����AlCl3��Һ��������������Al
A.0��B.1��C.2��D.3��
���𰸡�A
��������
![]() ��Ԫ�صĻ��ϼ�û�з����仯������������ԭ��Ӧ����
��Ԫ�صĻ��ϼ�û�з����仯������������ԭ��Ӧ����![]() ����
����
![]() ��Ԫ�صĻ��ϼ�Ϊ
��Ԫ�صĻ��ϼ�Ϊ![]() ��
��![]() �ۣ�
�ۣ�![]() ����д��
����д��![]() ����ʽ����ǦԪ�ػ��ϼ�Ϊ
����ʽ����ǦԪ�ػ��ϼ�Ϊ![]() ��
��![]() �ۣ�
�ۣ�![]() Ӧ��д��
Ӧ��д��![]() ����ʽ����
����ʽ����![]() ����
����
![]() ��������ø���¶Ƚϸ�ʱʧȥ���ԣ����������ã���
��������ø���¶Ƚϸ�ʱʧȥ���ԣ����������ã���![]() ����
����
![]() ͭ�������������
ͭ�������������![]() ��Һ��������Cu�������ǻ��ý��������
��Һ��������Cu�������ǻ��ý��������![]() ��Һ�����ϲ�������Al��������������
��Һ�����ϲ�������Al��������������![]() ����
����
��ѡ��A��

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�����Ŀ���Ȼ�������SOCl2��������ҽҩ��ũҩ��Ⱦ�Ϲ�ҵ��Ҳ�����л��ϳɹ�ҵ�����Ȼ�������֪��SOCl2������������±���ʾ��
��ɫ��״̬ | �۵� | �е� | ��ʴ�� | ˮ�� |
��ɫ����Һ�� | -105�� | 78�� | ǿ | ����ˮ�� |
��������ͼװ���Ʊ�SOCl2��
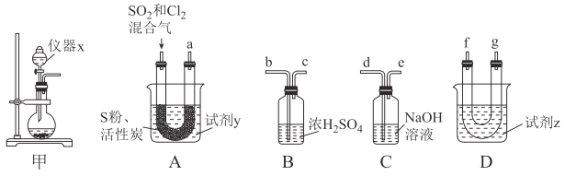
��ش��������⣺
���Ʊ�SO2��Cl2��
��1����ʵ��ѡ��װ�ü��Ʊ�SO2��Cl2��װ�ü�������x������Ϊ___������KMnO4��Ũ���ᷴӦ�Ʊ�Cl2����Ӧ�����ӷ���ʽΪ___��
���Ʊ�SOCl2��
�Ի���̿��Ϊ������SO2��C12���Ժ�S����180~200��ʱ��Ӧ�ϳ�SOCl2��ѡ��װ��A��B��C��D�����Ʊ����г֡�����װ����ȥ����
��2�������������ҵķ���װ��A��B��C��D������˳��Ϊ___���������ӿڵ���ĸ��ţ���
��3���Լ�yΪ___����ѡ����ĸ����ͬ�����Լ�zΪ___��
A����ˮ B���Ҵ� C��ʯ���� D����ˮ
��4��װ��A��U�ι��ڷ�����Ӧ�Ļ�ѧ����ʽΪ___��
��5��װ��C������Ϊ___����װ��A��ͨ���SO2��Cl2�����ʵ���֮��Ϊ1��3����װ��C�����ɵ���Ϊ___���ѧʽ���������ʵ����֤װ��C�����ɵ����к���SO42-��____��
����Ŀ������������������۷���Ϣ�صijɷ�֮һ�������㽶����ζ��ʵ�����Ʊ������������ķ�Ӧ��װ��ʾ��ͼ���й��������£�
![]()
��Ŀ | ��Է������� | �ܶ�/(g��cm3) | �е�/�� | ˮ���ܽ��� |
���촼 | 88 | 0.8123 | 131 | �� |
���� | 60 | 1.0492 | 118 | �� |
���������� | 130 | 0.8670 | 142 | ���� |
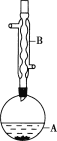
ʵ�鲽�裺
��A�м���4.4g���촼��6.0g���ᡢ����Ũ�����2��3Ƭ���Ƭ����ʼ��������A������50min����ӦҺ�������º����Һ©���У��ֱ�������ˮ������̼��������Һ��ˮϴ�ӣ��ֳ��IJ������������ˮMgSO4���壬����Ƭ�̣����˳�ȥMgSO4���壬�����������ռ�140��143����֣�������������3.9g��
�ش��������⣺
��1������B��������___��
��2����ϴ�Ӳ����У���һ��ˮϴ�͵ڶ���ˮϴ����ҪĿ�ķֱ���___��
��3����ϴ�ӡ���Һ�����У�Ӧ�����Ȼ���ã����ֲ��__(����)��
a.ֱ�ӽ������������ӷ�Һ©�����Ͽڵ���
b.ֱ�ӽ������������ӷ�Һ©�����¿ڷų�
c.�Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��������������¿ڷų�
d.�Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��������������Ͽڵ���
��4��ʵ���м���������ˮMgSO4��Ŀ����___��
��5����ʵ��IJ�����___(����)��
a.30% b.40% c.60% d.90%
����Ŀ����1���±��Dz�ͬ�¶���ˮ�����ӻ����ݣ�
�¶�/�� | 25 | T1 | T2 |
Kw/ mol2��L-2 | 1��10-14 | a | 1��10-12 |
�Իش����¼������⣺
����֪25��< T1< T2 ����T1ʱ��ˮ�� c(H+)______c(OH-)�� ��a_____1��10-14������<������>������=������
����T2�£���pH=1��H2SO4��ҺV1 L��pH=11��NaOH��ҺV2 L��ϣ����Ϻ���Һ���Ϊԭ����Һ���֮�ͣ�������Һ��pH=2����V1�UV2 =_________��
��2�������£���0.01 mol/L��CH3COOH��Һ���Va��0.01 mol/L��NaOH��Һ���Vb��ϣ���֪��CH3COOHϡ��Һ�ĵ���ƽ�ⳣ��ΪKa=2��10-5��
a. Va=Vbʱ���������Ϻ�����ҺΪ���ԣ�ԭ���ǣ�_____�������ӷ���ʽ���ͣ�����ƽ���ƽ�ⳣ��Kh=_________��
b.��Ϻ���Һ��pH=7����Va ____Vb������<������>������=�������û����Һ������Ũ���ɴ�С�����У�______��
c.������������pH=11��CH3COONa��Һ����ˮ�������c(OH-)=___________��