��Ŀ����
����Ŀ���Ȼ�������SOCl2��������ҽҩ��ũҩ��Ⱦ�Ϲ�ҵ��Ҳ�����л��ϳɹ�ҵ�����Ȼ�������֪��SOCl2������������±���ʾ��
��ɫ��״̬ | �۵� | �е� | ��ʴ�� | ˮ�� |
��ɫ����Һ�� | -105�� | 78�� | ǿ | ����ˮ�� |
��������ͼװ���Ʊ�SOCl2��
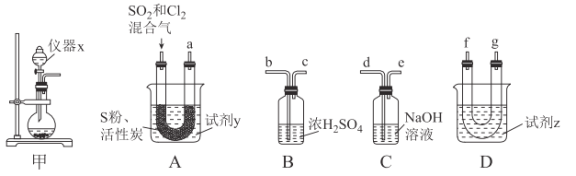
��ش��������⣺
���Ʊ�SO2��Cl2��
��1����ʵ��ѡ��װ�ü��Ʊ�SO2��Cl2��װ�ü�������x������Ϊ___������KMnO4��Ũ���ᷴӦ�Ʊ�Cl2����Ӧ�����ӷ���ʽΪ___��
���Ʊ�SOCl2��
�Ի���̿��Ϊ������SO2��C12���Ժ�S����180~200��ʱ��Ӧ�ϳ�SOCl2��ѡ��װ��A��B��C��D�����Ʊ����г֡�����װ����ȥ����
��2�������������ҵķ���װ��A��B��C��D������˳��Ϊ___���������ӿڵ���ĸ��ţ���
��3���Լ�yΪ___����ѡ����ĸ����ͬ�����Լ�zΪ___��
A����ˮ B���Ҵ� C��ʯ���� D����ˮ
��4��װ��A��U�ι��ڷ�����Ӧ�Ļ�ѧ����ʽΪ___��
��5��װ��C������Ϊ___����װ��A��ͨ���SO2��Cl2�����ʵ���֮��Ϊ1��3����װ��C�����ɵ���Ϊ___���ѧʽ���������ʵ����֤װ��C�����ɵ����к���SO42-��____��
���𰸡���Һ©�� 2MnO4-+16H++10Cl-=2Mn2++5Cl2��+8H2O afg��gf��bcde C D SO2+2Cl2+S![]() 2SOCl2 β��������������δ��Ӧ��SO2��Cl2�� NaC1��NaClO��Na2SO4 ȡװ��C��������Һ���Թ��У���ϡ�����ữ�����������ɣ������μ�BaCl2��Һ�����ɰ�ɫ������֤����SO42-
2SOCl2 β��������������δ��Ӧ��SO2��Cl2�� NaC1��NaClO��Na2SO4 ȡװ��C��������Һ���Թ��У���ϡ�����ữ�����������ɣ������μ�BaCl2��Һ�����ɰ�ɫ������֤����SO42-
��������
��.��1��װ�ü�������x������Ϊ��Һ©������ѡ��װ�ü��Ը�����غ�Ũ���ᷴӦ�Ʊ�Cl2����Ӧ�����ӷ���ʽΪ2MnO4-+16H++10Cl-=2Mn2++5Cl2��+8H2O��
��.��2�������������ҵķ���AΪ�Ʊ�װ�ã�DΪ�ռ�װ�ã�BΪ����װ�ã�������D���ֹC�е�ˮ�������룬CΪβ������װ�ã�����˳��Ϊafg��gf��bcde��
��3���Լ�yΪʯ���ͣ��ܱ�����ԡ�¶�Ϊ180��200�棻�Լ�zΪ��ˮ�������ռ����ɵ��Ȼ�������
��4��װ��A��U�ι��ڷ�����Ӧ�Ļ�ѧ����ʽΪSO2+2Cl2+S![]() 2SOCl2��
2SOCl2��
��5��װ��C������Ϊ����δ��Ӧ��SO2��Cl2����װ��A��ͨ���SO2��Cl2�����ʵ���֮��Ϊ1��3��Cl2��������װ��C�з����ķ�ӦΪSO2+Cl2+4NaOH===2NaCl+Na2SO4+2H2O��Cl2+2NaOH===NaCl+NaClO+H2O�����ɵ���ΪNaCl��Na2SO4��NaClO����֤װ��C�����ɵ����к���![]() �ľ������Ϊȡװ��C��������Һ���Թ��У���ϡ�����ữ�����������ɣ������μ�BaCl2��Һ�����ɰ�ɫ������֤����SO42-��
�ľ������Ϊȡװ��C��������Һ���Թ��У���ϡ�����ữ�����������ɣ������μ�BaCl2��Һ�����ɰ�ɫ������֤����SO42-��

 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�



