��Ŀ����
14���ȽϷ��ǻ�ѧ���о��������ʵĻ�������֮һ�������ñȽϷ�������⣮Na2O2�����������еij�����̬�ǽ��������ﷴӦ����2Na2O2+2CO2�T2Na2CO3+O2��Na2O2+CO=Na2CO3����1��ͨ���ȽϿ�֪�����ǽ���Ԫ�ش�������ϼۼ�ʱ������������Na2O2��Ӧ��O2���ɣ�
��2���Էֱ�д��Na2O2��SO2��SO3��Ӧ�Ļ�ѧ����ʽ��Na2O2+SO2=Na2SO4��2Na2O2+2SO3=2Na2SO4+O2��
��3���裨CN��2�Ļ�ѧ���ʺ�±��X2��X-Cl��Br��I�������ƣ���ѧ�ϳ�Ϊ��±�أ���д����CN��2��ˮ��Ӧ�Ļ�ѧ����ʽ��CN��2+H2O=HCN+HCNO��
���� ��1�����ݶ�����̼��һ����̼��̼Ԫ�ػ��ϼ۽�Ϸ�Ӧ����ʽ���
��2���������������������������ԭ��Ӧ���������ƣ������������������������Ϸ�Ӧ���������ƺ�������
��3���裨CN��2�Ļ�ѧ���ʺ�±��X2��X-Cl��Br��I�������ƣ���ˮ��Ӧ����HCN��HCNO��
��� �⣺��1��������̼��̼Ԫ�ش���+4�ۣ�CO��CԪ�ش���+2�ۣ�����2Na2O2+2CO2�T2Na2CO3+O2��Na2O2+CO=Na2CO3��֪�����ǽ���Ԫ�ش������̬������������Na2O2��Ӧ��O2���ɣ�
�ʴ�Ϊ������ϣ�
��2���������������������������ԭ��Ӧ���������ƣ�����ʽΪ��SO2+Na2O2=Na2SO4��
�����������������������Ϸ�Ӧ���������ƺ�����������ʽΪ��2SO3+2Na2O2=2Na2SO4+O2����
�ʴ�Ϊ��SO2+Na2O2=Na2SO4��2SO3+2Na2O2=2Na2SO4+O2����
��3���裨CN��2�Ļ�ѧ���ʺ�±��X2��X-Cl��Br��I�������ƣ���ˮ��Ӧ����HCN��HCNO���䷴Ӧ�ķ���ʽΪ����CN��2+H2O=HCN+HCNO��
�ʴ�Ϊ����CN��2+H2O=HCN+HCNO����3����CN��2+H2O=HCN+HCNO��1�֣���д�ɿ������Ҳ���֣�
���� ���⿼��������ԭ��Ӧ����ѧ����ʽ����д����ȷ�����Ļ�ѧ��Ӧ�ǽ����Ĺؼ�����ע�ⳣ��Ԫ�صĻ��ϼ۱仯����Ŀ�ϼ�

| A�� | 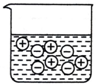 | B�� | 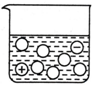 | C�� | 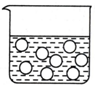 | D�� | 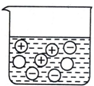 |
| A�� | ��ҵ�ƽ����ƣ���ⱥ��ʳ��ˮ | |
| B�� | ��ҵ���壺ijЩֲ���и�������������Ӻ���Ʒ����ȡ���ǹ�ҵ�ϻ�ȡ�����Ҫ;�� | |
| C�� | ұ���������Al2O3��ͬʱ�������ʯ��Na3AlF6����Ŀ����Ϊ�˽���Al2O3�����¶� | |
| D�� | ���Ṥҵ�����������������£��ӽӴ��ҽ����������������в����ܺ���SO2 |
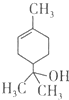
| A�� | 1mol����������ܺ�lmol���������ӳɷ�Ӧ | |
| B�� | ������������ | |
| C�� | ��������ʹ��ˮ��ɫ | |
| D�� | ����ʽΪC9H16O |
��1��ѡȡ��Ҫ��ʵ��װ�ã���ȷ������˳��ΪCAB������ţ���
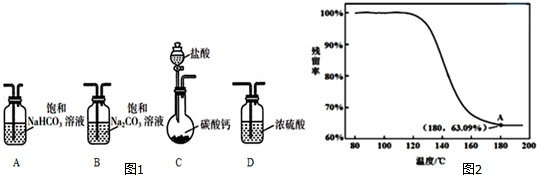
��2��Ϊȷ���ƵõĹ�����Ʒ�Ǵ�����NaHCO3��С��ͬѧ�������ʵ�鷽����
����������Ʒ��Һ�뱥�ͳ���ʯ��ˮ��Ӧ���۲�����
�ҷ���������Ʒ��Һ��BaCl2��Һ��Ӧ���۲�����
���������ⶨpH����
�����������ط�������
����������������������������
��Ϊ�ж��ҷ����Ŀ����ԣ�ijͬѧ�ô�����NaHCO3���Ƶ���Һ����BaCl2��Һ�������Ͻ���ʵ�飬������£�
NaHCO3��Һ BaCl2Ũ�� | 0.2mol•L-1 | 0.1mol•L-1 | 0.02mol•L-1 |
| 0.2mol•L-1 | ���� | ���� | �������� |
| 0.1mol•L-1 | ���� | �������� | ������ |
| 0.02mol•L-1 | �������� | ������ | ������ |
[��֪��0��l mol•L-1 NaHCO3��Һ�������c��CO32-��Ϊ0.001l mol•L-1��Ksp��BaCO3��=5.1��10-9]
��Qc=c��Ba2+����c��CO32-��=$\frac{0.2}{2}$��0.0011=1.1��10-4��5.1��10-9��
��ii���������ǣ�������������������ӷ���ʽBa2++2HCO3-=BaCO3��+CO2��+H2O��
����pH�Ʋⶨ�ı��������£�
ȡm�˵Ĺ��������ܽ���ˮ���V mL����Һ����pH�Ʋ�pH��
��Ӧ�����ʵ���ǣ���ȡ�������ķ�����NaHC03����ˮ�����V mL����Һ����pH�Ʋ�pH
�ܽ��ж�����ʵ�飬�õ�������������¶ȱ仯��������ͼ2��ʾ������A������õ��Ľ������ƵõĹ�����Ʒ�Ǵ�����NaHCO3��
��������=$\frac{ʣ����������}{ԭʼ���������}$��100%��
| A�� | ���³�ѹ�£�0.1mol D216O�к�������������������������ΪNA | |
| B�� | �Ȼƽ��18O2����ͨ��16O2�����ֲ�ͬ�ĺ��� | |
| C�� | ��״���£�2.24LCl2����ˮ��ת�Ƶĵ�����ĿΪ0.1NA | |
| D�� | 1L2mol•L-1��Al��NO3��3��Һ�к�Al3+����Ϊ2NA |

 ��
�� ��
��
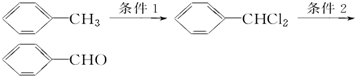
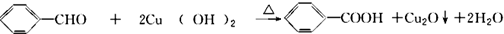 ��
��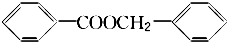 ��
��