��Ŀ����
����Ŀ����������������Ҫ�Ĺ���Ԫ�ء�
(1)��λ��Ԫ�����ڱ��������ڢ��壬���̬ԭ����δ�ɶԵ��Ӹ���Ϊ___________��
(2)��̬Fe3+�ĺ�������Ų�ʽ___________
(3)��������һ�ִ��Բ��ϣ���ҵ���Ʊ�ʱ������ˮ�ⷨ���Ʊ�ʱ����������(CO(NH2)2)�������Ƶȼ������ʡ����ط����������ǽ���Ԫ�صĵ縺���ɴ�С��˳����___________����������������������Ŀ֮��Ϊ___________����������̼ԭ�ӵ��ӻ�����___________��
(4)������Ҳ��ʹ�ó��������Ʊ�ʱ�����백(NH3)������(N2H4)�������֪��(NH3�۵㣺��77.8%�桢�е㣺��33.5%��)������(N2H4�۵㣺2�桢�е㣺113.5��C)�������۷е�ߵ͵���Ҫԭ��______________________��
(5)Co(NH3)5BrSO4���γ������ܵ�������֪Co3+����λ��Ϊ6��Ϊȷ����������Ľṹ���ֶ�����������������ʵ�飺�ڵ�һ���������Һ�м�����������Һ������ɫ���������һ������������Ϊ___________���ڵڶ����������Һ�м�����������Һ��������ɫ��������ڶ�������������Ϊ___________��

(6)��������̼�ܽ���r-Fe���γɵ�һ�ּ�϶�����壬���ԣ��侧��Ϊ���������ṹ������ͼ��ʾ��������ʵĻ�ѧʽΪ___________����Ʒ���ܶ�Ϊdg��cm��3���������������̼ԭ�ӵľ���Ϊ___________pm(�����ӵ�������ֵ��NA��ʾ��д����ļ���ʽ����)��
���𰸡�3 1s22s22p63s23p63d5����[Ar]3d5�� O>N>C>H 7:1 sp2 ��sp3 �������Ӽ��γ��������Ŀ���ڰ������γɵ���� NH3��Br- NH3�� SO42- FeC��Fe4C4�� ![]()
��������
��1����������Ԫ�����ڱ���λ�ã����������Ų�ʽ�ж�δ�ɶԵ��Ӹ�����
��2�����ݻ�̬Fe�ĺ�������Ų�ʽ�ж�ʧȥ�����3�����Ӻ�ĵ����Ų�ʽ��
��3��CO(NH2)2������C��N��H��Oԭ�ӹ��ɣ�����ͬһ����Ԫ�ص縺����������ͬһ����Ԫ�ص縺����С�Ĺ��ɽ��з��������۵�����1���Ҽ�������˫����1���Ҽ���1���м�����ϳɼ����ɼ�����ӽṹʽ�������𣻴����ƺ�������̼��˫��������ӻ�������۽��з�����
��4��������������ʵ��۷е��Ӱ�죻
��5�������������ڽ�֮��ͨ���γ����Ӽ����������ӿ��Ե��룬���ڽ�������������Ե��롣
��1������27��Ԫ�أ�λ��Ԫ�����ڱ��������ڢ��壬���̬ԭ�ӵĵ����Ų�ʽΪ��1s22s22p63s23p63d74s2������3d�ܼ���ԭ�ӹ������3��δ�ɶԵ��ӣ�
�ʴ�Ϊ��3��
��2����Feԭ������Ϊ26��λ�ڵ������ڢ��壬���̬ԭ�ӵĵ����Ų�ʽΪ��1s22s22p63s23p63d64s2�����̬Fe3+�ĺ�������Ų�ʽ1s22s22p63s23p63d5����[Ar]3d5����
�ʴ�Ϊ��1s22s22p63s23p63d5����[Ar]3d5����
��3��ͬһ�����д�����Ԫ�صĵ縺����������ͬһ����Ԫ�ش��ϵ��µ縺����С����C��N��H��O�ĵ縺���ɴ�С��˳����O>N>C>H��CO(NH2)2�Ľṹ��ʽΪ��![]() ���ɿ��������ڹ���7���Ҽ���1���м�����Ҽ���м��ĸ�����Ϊ7:1����������ӵ�����̼ԭ�Ӳ��ṩ�¶Ե��ӣ��������������̼ԭ�Ӵ�������λ��,��̼ԭ��sp3�ӻ�,�Ȼ�̼ԭ��sp2�ӻ��������ӻ�����Ϊsp2 ��sp3��
���ɿ��������ڹ���7���Ҽ���1���м�����Ҽ���м��ĸ�����Ϊ7:1����������ӵ�����̼ԭ�Ӳ��ṩ�¶Ե��ӣ��������������̼ԭ�Ӵ�������λ��,��̼ԭ��sp3�ӻ�,�Ȼ�̼ԭ��sp2�ӻ��������ӻ�����Ϊsp2 ��sp3��
�ʴ�Ϊ��O>N>C>H��7:1��sp2 ��sp3��
��4��N2H4��NH3��ȣ�����Ӽ�������Ŀ�ϰ������γɵ�����࣬��N2H4�۷е��������ߣ�
�ʴ�Ϊ���������Ӽ��γ��������Ŀ���ڰ������γɵ������
��5��Co(NH3)5BrSO4���γ������ܵ�������֪Co3+����λ��Ϊ6���ڵ�һ���������Һ�м���������Һ������ɫ��������ɫ��������������˵����һ�����岻�����������������������ӣ������������ڽ�����壬���һ������������ΪNH3��Br-���ڵڶ����������Һ�м�����������Һ��������ɫ�����������Ϊ�廯���������岻������Br-������һ������������ΪNH3��SO42-��
�ʴ�Ϊ��NH3��Br-��NH3��SO42-��
��6�����ݾ����ṹ��֪����������ԭ�Ӹ���Ϊ8��1/8+6��1/2 = 4��̼ԭ�Ӹ���Ϊ1+12��1/4 = 4����˸����ʵĻ�ѧʽΪFeC��Fe4C4�������������������̼ԭ��֮��ľ���Ϊ��Խ��ߵ�һ�룬�����ı߳� = ![]() =
= ![]() cm������Խ���Ϊ
cm������Խ���Ϊ![]() cm��
cm��![]() �����Ծ����������̼ԭ��֮��ľ��� =
�����Ծ����������̼ԭ��֮��ľ��� =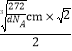 =
= ![]() pm��
pm��
�ʴ�Ϊ��FeC��Fe4C4����![]() pm��
pm��

����Ŀ��N2O5��һ����������������һ���¶��¿ɷ������·�Ӧ��2N2O5(g) ![]() 4NO2(g)��O2(g)��H>0��T1�¶�ʱ�����ܱ�������ͨ��N2O5������ʵ�����ݼ��±���
4NO2(g)��O2(g)��H>0��T1�¶�ʱ�����ܱ�������ͨ��N2O5������ʵ�����ݼ��±���
ʱ��/s | 0 | 500 | 1000 | 1500 |
c(N2O5)mol/L | 5.00 | 3.53 | 2.50 | 2.50 |
����˵���в���ȷ���ǣ�
A. T1�¶��£�500sʱO2��Ũ��Ϊ0.74mol/L
B. ƽ������������䣬�����������ѹ����ԭ����1/2������ƽ��ʱc(N2O5)>5.00 mol/L
C. T1�¶��µ�ƽ�ⳣ��ΪK1��T2�¶��µ�ƽ�ⳣ��ΪK2����T1>T2����K1<K2
D. T1�¶��µ�ƽ�ⳣ��ΪK1=125��ƽ��ʱN2O5��ת����Ϊ0.5




