��Ŀ����
����Ŀ��N2O5��һ����������������һ���¶��¿ɷ������·�Ӧ��2N2O5(g) ![]() 4NO2(g)��O2(g)��H>0��T1�¶�ʱ�����ܱ�������ͨ��N2O5������ʵ�����ݼ��±���
4NO2(g)��O2(g)��H>0��T1�¶�ʱ�����ܱ�������ͨ��N2O5������ʵ�����ݼ��±���
ʱ��/s | 0 | 500 | 1000 | 1500 |
c(N2O5)mol/L | 5.00 | 3.53 | 2.50 | 2.50 |
����˵���в���ȷ���ǣ�
A. T1�¶��£�500sʱO2��Ũ��Ϊ0.74mol/L
B. ƽ������������䣬�����������ѹ����ԭ����1/2������ƽ��ʱc(N2O5)>5.00 mol/L
C. T1�¶��µ�ƽ�ⳣ��ΪK1��T2�¶��µ�ƽ�ⳣ��ΪK2����T1>T2����K1<K2
D. T1�¶��µ�ƽ�ⳣ��ΪK1=125��ƽ��ʱN2O5��ת����Ϊ0.5
���𰸡�C
��������A��500s����N2O5�����ʵ���Ũ��Ϊ(5��3.53)mol��L��1=1.47mol��L��1�������������ʵ���Ũ��Ϊ1.47/2mol��L��1=0.735mol��L��1����A˵����ȷ��B�����ѹ����ԭ����1/2������ƽ�ⲻ�ƶ���N2O5��Ũ��Ϊ5mol��L��1��ѹǿ����ƽ�����淴Ӧ�����ƶ���N2O5Ũ����������5mol��L��1����B˵����ȷ��C���˷�Ӧ�����ȷ�Ӧ�������¶ȣ�ƽ�����淴Ӧ�����ƶ�����K1>K2����C˵������D��2N2O5(g) ![]() 4NO2(g)��O2(g)
4NO2(g)��O2(g)
��ʼ�� 5 0 0
�仯�� 2x 4x x
ƽ�⣺ 5��2x 4x x ���ݻ�ѧƽ�ⳣ�����壬K= 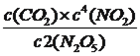 =
=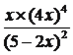 =125�����2x=2.5��N2O5��ת����Ϊ2.5/5=0.5��
=125�����2x=2.5��N2O5��ת����Ϊ2.5/5=0.5��