��Ŀ����
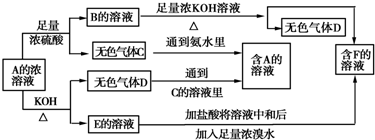
17�� ��100mL NaOH��Һ����ͨ��һ������CO2��������Һ������䣩����������Һ��pH��7��
��100mL NaOH��Һ����ͨ��һ������CO2��������Һ������䣩����������Һ��pH��7����1����ʱ��Һ����������ǵ�һ�ɷ֣�������Na2CO3��NaHCO3������Ƕ��ֳɷ֣�������Na2CO3��NaHCO3��NaOH��Na2CO3��
��2�����ȡ����Һ10mL����ϡ����100mL�������ϡ�ͺ����Һ����μ���0.1mol/L�����ᣬ����CO2������������״���£��������������������ϵ��ͼ��ʾ��
��д��OA����������Ӧ�����ӷ���ʽ��OH-+H+�TH2O��CO32-+H+�THCO3-��
��NaOH������CO2��������Һ������ΪNaOH��Na2CO3�������ʵ���Ũ��֮��Ϊ1��1��
�۲�����CO2�������״���£�Ϊ0.56L��
��ԭNaOH��Һ�����ʵ���Ũ��Ϊ0.75mol/L��
���� ��1��CO2��NaOH��Ӧ������Na2CO3��NaHCO3�������NaOHʣ��ʱ��ֻ����Na2CO3��Na2CO3��NaHCO3��Һ���ʼ��ԣ�
��2����BC�η�����NaHCO3+HCl�TNaCl+H2O+CO2������OA�����ĵ�����ΪA��B���ĵ�����Ķ�������ΪNaOH��Na2CO3�Ļ���OA����������Ӧ��NaOH+HCl=NaCl+H2O��Na2CO3+HCl=NaHCO3+NaCl��
���ɷ���ʽ��֪��Na2CO3̼����ת��ΪNaHCO3�������������NaHCO3��ȫ��Ӧ����CO2�������������ȣ����Լ���NaOH�����������������������̼���Ƶ����ʵ���֮�ȵ��������������֮�ȣ�
�۸���n=cV����AB������HCl���ʵ��������ݷ���ʽ�������ɶ�����̼���ʵ������ٸ���V=nVm���������
��B������ΪNaCl�����������ӡ��������غ��У�n��HCl��=n��NaCl��=n��NaOH�����ٸ���c=$\frac{n}{V}$����ԭNaOH��Һ�����ʵ���Ũ�ȣ�
��� �⣺��1��CO2��NaOH��Ӧ������Na2CO3��NaHCO3��������Һ���ʼ��ԣ�������ǵ�һ�ɷ֣�������Na2CO3��NaHCO3������Ƕ��ֳɷ֣������ǣ�Na2CO3��NaHCO3��NaOH��Na2CO3��
�ʴ�Ϊ��Na2CO3��NaHCO3��Na2CO3��NaHCO3��NaOH��Na2CO3��
��2����BC�η�����NaHCO3+HCl�TNaCl+H2O+CO2������OA�����ĵ�����ΪA��B���ĵ�����Ķ�������ΪNaOH��Na2CO3�Ļ���OA����������Ӧ��NaOH+HCl=NaCl+H2O��Na2CO3+HCl=NaHCO3+NaCl����Ӧ�����ӷ���ʽ�ֱ�Ϊ��OH-+H+�TH2O��CO32-+H+�THCO3-��
�ʴ�Ϊ��OH-+H+�TH2O��CO32-+H+�THCO3-��
���ɢ��з�����֪����Һ������ΪNaOH��Na2CO3��
���ݷ�Ӧ����ʽ��CO32-+H+�THCO3-��HCO3-+H+=CO2��+H2O����֪Na2CO3̼����ת��ΪNaHCO3�������������NaHCO3��ȫ��Ӧ����CO2�������������ȣ���Ϊ25mL���������������ĵ��������Ҳ��50mL-25mL=25mL����Na2CO3��NaOH�����ʵ�����ȣ������ʵ���Ũ��֮��Ϊ1��1��
�ʴ�Ϊ��NaOH��Na2CO3��1��1��
��AB������HCl���ʵ���Ϊ0.1mol/L��0.025L=0.0025mol����HCO3-+H+=CO2��+H2O����֪���ɶ�����̼Ϊ0.0025mol�������Ϊ22.4L/mol��0.0025mol=0.56L��
�ʴ�Ϊ��0.56L��
��B������ΪNaCl�����������ӡ��������غ��У�n��NaOH��=n��NaCl��=n��HCl��=0.1mol/L��0.075L=0.0075mol��ԭNaOH��Һ�����ʵ���Ũ��Ϊ$\frac{0.0075mol}{0.01L}$=0.75mol/L��
�ʴ�Ϊ��0.75mol/L��
���� ���⿼������ļ��㣬��Ŀ�Ѷ��еȣ�����ؼ��Ǹ���ͼ���ж���Һ���ʵijɷ֣�ע�����������غ�˼���Ӧ�ã�

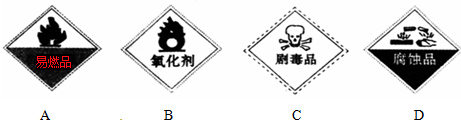
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | 1��17 | B�� | 15��8 | C�� | 14�� 6 | D�� | 12��9 |
| A�� | CH3COOH | B�� | H2 | C�� | HCOOH | D�� | CO |

| A�� | �����ʿ�����Ϊ���� | B�� | ���л���ķ���ʽΪC19H32O | ||
| C�� | ��ʹ������Ȼ�̼��Һ��ɫ | D�� | �����ʷ����е�����̼ԭ�Ӿ����� |
| A�� | Ǧ�������ڶ��ε�� | |
| B�� | ����������ʴ�̲�������Ϊ�����ǿ���� | |
| C�� | Ϊ�˷�ֹʳƷ�ܳ�������ʳƷ��װ���з���������� | |
| D�� | �����ƹ��µĹ��ά�������л��߷��Ӳ��� |
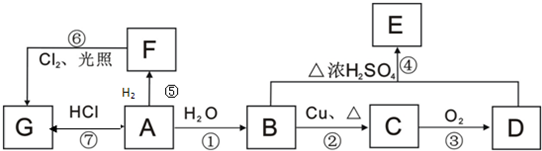
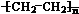 ��A��ˮ��һ�������·�Ӧ����B��BΪ�����г������л��B������һ�������г����л�����һ�������·�Ӧ��������ζ������C��д������C�Ļ�ѧ����ʽ
��A��ˮ��һ�������·�Ӧ����B��BΪ�����г������л��B������һ�������г����л�����һ�������·�Ӧ��������ζ������C��д������C�Ļ�ѧ����ʽ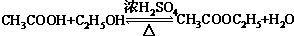 �������У����˶�Ա������˻�Ť��ʱ�����ҽ���������˲�λ���������飨�е�12.27�棩���оֲ��䶳����Ӧ����������ϩ���Ȼ�����һ���������Ƶ�������Ļ�ѧ��Ӧ����ʽ�ǣ��л����ýṹ��ʽ��ʾ��CH2=CH2+HC1
�������У����˶�Ա������˻�Ť��ʱ�����ҽ���������˲�λ���������飨�е�12.27�棩���оֲ��䶳����Ӧ����������ϩ���Ȼ�����һ���������Ƶ�������Ļ�ѧ��Ӧ����ʽ�ǣ��л����ýṹ��ʽ��ʾ��CH2=CH2+HC1 CH3CH2C1���÷�Ӧ�����Ǽӳɷ�Ӧ��
CH3CH2C1���÷�Ӧ�����Ǽӳɷ�Ӧ��