��Ŀ����
8���ס��ҡ������������ֶ�����Ԫ�أ���ԭ�����������Ӳ㣬��һ����������������ȣ���ԭ�ӵĺ������������ԭ�Ӻ����������1����ԭ�ӵ������������Ǵ�����������2������ԭ�Ӻ˵�����ȱ�ԭ�Ӻ˵������2����ش���1������NaԪ�أ���Ԫ�ط��ţ����ĵ�����ˮ��Ӧ�Ļ�ѧ����ʽΪ2Na+2H2O=2NaOH+H2����
��2������MgԪ�أ���Ԫ�ط��ţ���ԭ�ӽṹʾ��ͼΪ
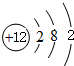 ��
����3������̼Ԫ�أ���Ԫ�����ƣ�������������Ӧˮ����Ļ�ѧʽ��H2CO3��
��4��������Ԫ�أ���Ԫ�����ƣ������붡Ԫ����ɵĻ������������ӻ����������ӻ�������ߡ����ۻ������
���� �ס��ҡ������������ֶ�����Ԫ�أ���ԭ�����������Ӳ㣬��һ����������������ȣ�������MgԪ�أ���ԭ�ӵĺ������������ԭ�Ӻ����������1�����ΪNaԪ�أ���ԭ�������������Ǵ�����������2����ԭ��ֻ����2�����Ӳ㣬����������Ϊ4���������CԪ�أ���ԭ�Ӻ˵�����ȱ�ԭ�Ӻ˵������2������OԪ�أ��ݴ˴��⣮
��� �⣺�ס��ҡ������������ֶ�����Ԫ�أ���ԭ�����������Ӳ㣬��һ����������������ȣ�������MgԪ�أ���ԭ�ӵĺ������������ԭ�Ӻ����������1�����ΪNaԪ�أ���ԭ�������������Ǵ�����������2����ԭ��ֻ����2�����Ӳ㣬����������Ϊ4���������CԪ�أ���ԭ�Ӻ˵�����ȱ�ԭ�Ӻ˵������2������OԪ�أ�
��1������NaԪ�أ�Na��ˮ��Ӧ����������������������Ӧ����ʽΪ��2Na+2H2O=2NaOH+H2�����ʴ�Ϊ��Na��2Na+2H2O=2NaOH+H2����
��2������MgԪ�أ�ԭ�ӽṹʾ��ͼΪ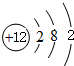 ���ʴ�Ϊ��Mg��
���ʴ�Ϊ��Mg��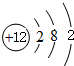 ��
��
��3������̼Ԫ�أ�����������Ӧˮ����Ļ�ѧʽ��H2CO3���ʴ�Ϊ��̼��H2CO3��
��4��������Ԫ�أ���ΪNa�����߿���ɵ�������Na2O��Na2O2�����������ӻ�����ʴ�Ϊ���������ӻ����
���� ���⿼��ԭ�ӽṹ��Ԫ�����ʣ�����ԭ�ӽṹȷ��Ԫ�أ���Ҫѧ���������պ�������Ų����ɣ������ڻ���֪ʶ�Ĺ��̣�

| ��� | �� | �� | �� |
| �� | CO2 | NH3 | CaCl2 |
| �� | CO2 | SO2 | ʯ��ˮ |
| �� | NO2 | SO2 | BaCl2 |
| �� | HCl | CO2 | ʯ��ˮ |
| A�� | �ڢۢ� | B�� | �٢ڢ� | C�� | �٢ۢ� | D�� | ȫ�� |
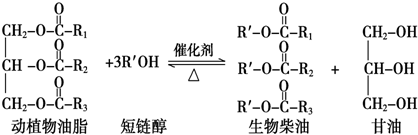
��������������ǣ�������
| A�� | ��������ɿ�������Դ�Ƶ� | B�� | ��������Dz�ͬ����ɵĻ���� | ||
| C�� | ��ֲ����֬�Ǹ߷��ӻ����� | D�� | ���ع��͡��������Ʊ�������� |
| A�� |  2-�һ����� 2-�һ����� | B�� | CH3CH2CH2CH2OH 1-���� | ||
| C�� |  �Զ��ױ� �Զ��ױ� | D�� |  2-��-2-��ϩ 2-��-2-��ϩ |
| A�� | ˫ԭ�ӷ����еĹ��ۼ���һ���ǷǼ��Լ� | |
| B�� | �κ�ԭ�ӵ�ԭ�Ӻ˶��������Ӻ����ӹ��ɵ� | |
| C�� | �Ǽ��Լ�Ҳ���ܴ��������ӻ������� | |
| D�� | BF3��CS2����������ԭ�ӵ�����㶼��8�����ȶ��ṹ |
| A�� | ú�ĸ����������仯 | |
| B�� | Һ��ʯ��������Ȼ������Ҫ�ɷֶ���̼�⻯���� | |
| C�� | ʯ���ѻ��õ��������Ǵ����� | |
| D�� | ú��������ʯ�͵ķ����������仯 |
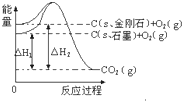
| A�� | C��sʯī��=C��s���ʯ����H=+1.9 kJ•mol-1 | |
| B�� | ���ʯ���ȶ��Դ���ʯī | |
| C�� | ʯī�ͽ��ʯת���������仯 | |
| D�� | 1 molʯī�������е���������1 mol���ʯ�������е���������1.9 kJ |
