��Ŀ����
����Ŀ������п�̳�����Ҫ�ɷ�ΪZnO��������CuO��FeO��Ϊԭ�ϣ�������ȡ����п�ͽ���п��
��.��ȡ����п��Ҫ�������£�
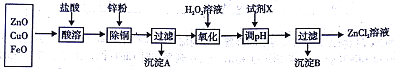
�±��г�����ؽ������������������������pH����ʼ������pH����������Ũ��Ϊl.0mol/L���㣩��
�������� | ��ʼ������pH | ������ȫ��pH |
Fe3+ | 1.1 | 3.2 |
Zn2+ | 5.2 | 6.4 |
Fe2+ | 5.8 | 8.8 |
��1��Ϊ�˼ӿ췴Ӧ������������Ҫ�ʵ����������¶Ȳ���̫�ߣ�ԭ����_________��
��2������H2O2��Һ������Ӧ�����ӷ���ʽ_______��
��3������ͼ����Ϊ�˽�����Һ����ȣ�����pH��ΧΪ_______�����Լ�XΪZn2(OH)2CO3������X�����ʵ����ӷ���ʽΪ________��
��4����֪�������£�Ksp[Fe(OH)3]=4.0��10-38����pH=3ʱ����Һ��c(Fe3+)Ϊ______����ZnCl2��Һ����ȡ��ˮZnCl2�ķ�����__________.
��.��ȡ����п���ü��ܽ�ZnO(s)+2NaOH (aq)+H2O=Na2[Zn(OH)4](aq),Ȼ�����ȡҺ��
��5����п�̳����ü��ܣ������������ܺ�������Ҫԭ����______��
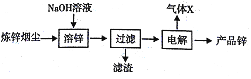
��6����ʯī���缫���ʱ����������������Ϊ______�������ĵ缫��ӦΪ______��
���𰸡� ��������ӷ� 2Fe2++H2O2+2H+=2Fe3++2H2O 3.2��PH<5.2 3Zn2(OH)2CO3+4Fe3++3H2O=4Fe(OH)3��+6Zn2++3CO2�� 4.0��10-5 ���Ȼ������壬�����Ȼ�п��Һ��������ʧȥ�ᾧˮ ����ͭ���������������ڼ���Һ�� O2���������� [Zn(OH)4]2-+2e-=Zn+4OH-
�����������������������Ҫ�������Ļ���������ʡ�
��1��Ϊ�˼ӿ췴Ӧ������������Ҫ�ʵ����ȣ����¶Ȳ���̫�ߣ���Ϊ������лӷ��ԣ�����ԭ���Ǽ�������ӷ���
��2������H2O2��Һ��Fe2+��������������Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2H+=2Fe3++2H2O ��
��3��Ϊ��ʹFe3+��ȫ������Zn2+��������������pH��ΧΪ3.2��PH<5.2 ��������Fe3+�������ʵ����ӷ���ʽΪ3Zn2(OH)2CO3+4Fe3++3H2O=4Fe(OH)3��+6Zn2++3CO2����
��4����pH=3ʱ��c(OH-)=![]() =
=![]() -mol/L =1��10-11mol/L�� Ksp[Fe(OH)3]= c(Fe3+)c3(OH-) = c(Fe3+)��(10-11)3=4.0��10-38��c(Fe3+)=4.0��10-5 mol/L��Ϊ������ZnCl2��ˮ�ⷴӦ����ZnCl2��Һ����ȡ��ˮZnCl2�ķ��������Ȼ��������У������Ȼ�п��Һ��������ʧȥ�ᾧˮ��
-mol/L =1��10-11mol/L�� Ksp[Fe(OH)3]= c(Fe3+)c3(OH-) = c(Fe3+)��(10-11)3=4.0��10-38��c(Fe3+)=4.0��10-5 mol/L��Ϊ������ZnCl2��ˮ�ⷴӦ����ZnCl2��Һ����ȡ��ˮZnCl2�ķ��������Ȼ��������У������Ȼ�п��Һ��������ʧȥ�ᾧˮ��
��. ��5����п�̳����ü��ܣ������������ܺ��⣬��Ҫԭ��������ͭ���������������ڼ���Һ�С�
��6����ʯī���缫���ʱ����������������Ϊ�����������ĵ缫��ӦΪ[Zn(OH)4]2-+2e-=Zn+4OH-��