��Ŀ����
��15�֣�����A��B��C��D��E��F��G��H���ֶ���������Ԫ�أ�ԭ����������������֪A��E��D��G�ֱ�ͬ���壻E��F��G��Hͬ���ڣ�A�ֱ���C��D���γɺ���10�����ӵĹ��ۻ�����M��N��B������������������Ӳ�����2����D�ǵؿ��к�������Ԫ�أ�Fλ��B��ǰһ���塣��ش��������⣺
��1��Ԫ��B�����ڱ��е�λ�� ��M�Ŀռ乹���� ��
��2��A��D��E����Ԫ�����һ�ֳ��������W��û�����������Ӿ�����ͬ��ԭ���������Ŀ�Ҳ����磬W�ĵ���ʽΪ ����ҵ������ijһ����Ӧ��ͬʱ�����û������H�ĵ��ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ ��
��3��E��FԪ�ص�����������Ӧ��ˮ����֮�䷴Ӧ�����ӷ���ʽ ��
��4��M��N���ܽ��H+�����н��H+������ǿ���� ���ѧʽ����N���H+���γɵ�������ԭ�Ӳ��� �ӻ�������DZ�N�еļ��Ǵ�ԭ��Ϊ ��
��5��E�ֱ���D��G�γ�Ħ��������ȵĻ�����X��Y������Y��ˮ��Һ�Լ��Ե�ԭ����
�������ӷ���ʽ��ʾ����������7.8 g X��ˮ��Ӧ�ų�Q kJ������Q��0����д���÷�Ӧ���Ȼ�ѧ����ʽ ��
��1����2���ڢ�A�壻 �����Σ�
��2��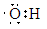
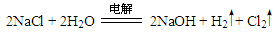
��3��Al(OH)3 + OH��==AlO2�� + 2H2O
��4��NH3��sp3��H2O��Oԭ����2�Թ¶Ե��ӣ�H3O+��Oԭ����1�Թ¶Ե��ӣ��ų���С
��5��S2��+ H2O HS�� + OH�� ��
HS�� + OH�� ��
2Na2O2(s) + 2H2O��1��="=4NaOH(aq)" + O2(g) ��H=��20Q kJ/mol
���������������������֪��A��B��C��D��E��F��G��H���ֶ���������Ԫ�أ�ԭ��������������B������������������Ӳ�����2������BΪ̼Ԫ�أ�D�ǵؿ��к�������Ԫ�أ���DΪ��Ԫ�أ�CΪ��Ԫ�أ�A�ֱ���C��D���γɺ���10�����ӵĹ��ۻ�����M��N����AΪ��Ԫ�أ�MΪNH3��NΪH2O��D��G�ֱ�ͬ���壬��GΪ��Ԫ�أ�HΪ��Ԫ�أ�A��Eͬ���壬��EΪ��Ԫ�أ�Fλ��B��ǰһ���壬��FΪ��Ԫ�ء���1��Ԫ��BΪ̼Ԫ�أ������ڱ��е�λ�õ�2���ڢ�A�壻MΪNH3���ռ乹���������Σ���2��A��D��E����Ԫ�����һ�ֳ����������������ƣ�W��û�����������Ӿ�����ͬ��ԭ���������Ŀ�Ҳ����磬WΪ�ǻ�������ʽ���𰸣���ҵ�ϵ�ⱥ���Ȼ�����Һ�����������ơ���������������ѧ����ʽΪ2NaCl+2H2O 2NaOH+H2��+Cl2������3���������ƺ�����������Ӧ����ƫ�����ƺ�ˮ����Ӧ�����ӷ���ʽΪAl(OH)3 + OH��==AlO2�� + 2H2O����4��NH3��H2O���ܽ��H+�����н��H+������ǿ����NH3��H3O+����ԭ�Ӳ���sp3�ӻ�,����DZ�H2O�еļ��Ǵ�ԭ��ΪH2O��Oԭ����2�Թ¶Ե��ӣ�H3O+��Oԭ����1�Թ¶Ե��ӣ��ų���С����5�����Ƶ�ˮ��Һ�Լ��Ե�ԭ����S2��+ H2O
2NaOH+H2��+Cl2������3���������ƺ�����������Ӧ����ƫ�����ƺ�ˮ����Ӧ�����ӷ���ʽΪAl(OH)3 + OH��==AlO2�� + 2H2O����4��NH3��H2O���ܽ��H+�����н��H+������ǿ����NH3��H3O+����ԭ�Ӳ���sp3�ӻ�,����DZ�H2O�еļ��Ǵ�ԭ��ΪH2O��Oԭ����2�Թ¶Ե��ӣ�H3O+��Oԭ����1�Թ¶Ե��ӣ��ų���С����5�����Ƶ�ˮ��Һ�Լ��Ե�ԭ����S2��+ H2O HS�� + OH�� ��������7.8 g X��ˮ��Ӧ�ų�Q kJ������Q��0�����÷�Ӧ���Ȼ�ѧ����ʽΪ2Na2O2(s) + 2H2O��1��="=4NaOH(aq)" + O2(g) ��H=��20Q kJ/mol��
HS�� + OH�� ��������7.8 g X��ˮ��Ӧ�ų�Q kJ������Q��0�����÷�Ӧ���Ȼ�ѧ����ʽΪ2Na2O2(s) + 2H2O��1��="=4NaOH(aq)" + O2(g) ��H=��20Q kJ/mol��
���㣺����Ԫ���ƶϼ�������ʵĽṹ�����ʣ��Ȼ�ѧ����ʽ����д��

��ѧ�ҽ�����Ԫ��Ǧ��봵�ԭ�Ӻ˶�ײ�������һ��������Ϊ118��������Ϊ293�ij���Ԫ�أ���Ԫ��ԭ�Ӻ��ڵ�����������������֮��Ϊ
| A��47 | B��57 |
| C��61 | D��175 |
�±��г��ˢ١���ʮ��Ԫ�������ڱ��е�λ�ã�
| �������� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | | | | �� | �� | | | |
| 3 | �� | �� | �� | �� | | �� | �� | �� |
| 4 | | | | | | | �� | |
�����л������ҪԪ��������������������������ʯ���������Ļ���Ԫ����������������������
��ѧ��������õ�Ԫ��������������������̬�⻯���ˮ��Һ�ʼ��Ե�Ԫ��������������������
�������١���Ԫ�ص�����������Ӧ��ˮ�����У�
������ǿ�����ʵĵ���ʽΪ������������������������ǿ�����ʵĻ�ѧʽΪ����������������
������Ԫ�آܺ͢ݵĵ���Ϊ�缫����Ԫ�آ۵�����������Ӧ��ˮ�����ˮ��Һ���ԭ��أ���ܵĵ����ڴ�ԭ�������______���������������
��Ԫ�آߵ�ij������Ϊ�д̼�����ζ����ɫ���壬���⻯��Ϊ�г�������ζ����ɫ���塣�������������ϣ�������һ�ֵ���ɫ��ĩ���˷�Ӧ��ѧ����ʽΪ�������������������������������������������������˷�Ӧ���������������Ϊ3��2g����Ӧ��ת�Ƶĵ�����Ϊ����������������������������ֵ����
��Ԫ�آ��Ԫ�آ����ߺ˵����֮����������������������Ԫ���зǽ����Խ�����Ԫ������������������Ԫ�����ƣ�����˵��������Ԫ�صķǽ�����ǿ����ʵ����ʵ�ǣ������ӷ���ʽ��ʾ��
����������������������������������������������������������������������������
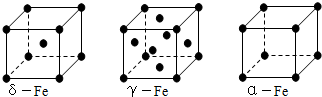
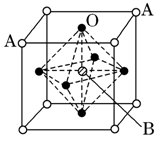
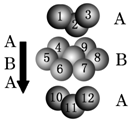
ij������A����TK���¾���Ļ����ṹ��Ԫ������ͼ��ʾ��T K����ת��Ϊ����ͼ��ʾ�ṹ�Ļ����ṹ��Ԫ�������־��������ڽ���Aԭ�Ӽ������ͬ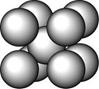 ��������
��������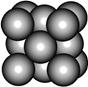
��1����T K���µĴ�A�����У���Aԭ�ӵȾ����������Aԭ����Ϊ______������T K���ϵĴ�A�����У���Aԭ�ӵȾ����������Aԭ����Ϊ___________��
��2����A�����ھ���ת��ǰ�������ṹ��Ԫ�ı߳�֮��Ϊ��TK������TK����֮�ȣ�___________��
��3������ͼ�ĵĶѻ���ʽΪ������������������ TK���¾��ⶨ��ṹ�����ʲ������±���ʾ
| ���� | ���ԭ������ | ���� | ԭ�Ӱ뾶/pm | �ܶ�/g���M-3 | ԭ�ӻ���/kJ��mol-1 |
| Na | 22.99 | s�� | 186 | 0.960 | 108.4 |
| A | 60.20 | d�� | r | 7.407 | 7735 |
��������������������������������������������������������������������������
����֪
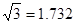 ��7.407��
��7.407��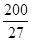 ,1pm=10
,1pm=10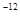 m��
m�� 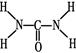 �������ط�����C��Nԭ�ӵ��ӻ���ʽ�ֱ��ǡ���
�������ط�����C��Nԭ�ӵ��ӻ���ʽ�ֱ��ǡ���