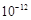��Ŀ����
�����й����ʳ��ӵķ����п��е���
| A����ȥʯӢ�л��е��������ۣ��ɼ�������������Һ�ܽ����� |
| B����ȥ���������л��е��������ᣬ���뱥������������Һ���Һ |
| C����֪��Ksp��CuS��<Ksp��FeS�����ɼ�����������ܵ����FeS��ʹˮ��������Cu2+ת���������������ȥ |
| D������������FeBr2��FeCl2��Һ�У�����������ˮ���ټ�CCl4��ȡ��Һ���Գ�ȥFeCl2��Һ�е�FeBr2 |
D
��������� A��ʯӢ�ɷ��Ƕ������裬������������Һ��Ӧ���ܽ⣬���� B����������������������Һ�з���ˮ�ⷴӦ������C�����ݳ����ܽ�ƽ��֪ʶ������ʵ�ִ����ܵĵ������ܵ�ת������ȷ�� D��Fe2+��ԭ��ǿ��Br����Cl2����������Fe2+������

��ϰ��ϵ�д�
�����Ŀ