��Ŀ����
�������ȼҵ�еķ�������Ҫ����þ�����������ƵȵĹ����κ�̼���Ρ�ʵ����������Ϊԭ����ȡMgSO4��7H2O���������£�

��֪��(��) Ksp[Mg(OH)2]��6.0��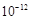
(��) Fe2+��Fe3+��Al3+��ʼ��������ȫ������pH��Χ����Ϊ��7.1~9.6��2.0~3.7��3.1~4.7
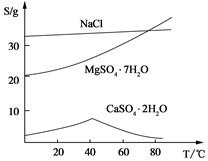
(��) ���ֻ�������ܽ�ȣ�S�����¶ȱ仯������ͼ��
�ش��������⣺
��1���������м�H2SO4��Һ����pHΪ1��2�Լ���һ����е�Ŀ���ǣ�������
��2������Һ��Mg2+��Ũ��Ϊ6 mol/L����ҺpH�������ſ��ܲ���Mg(OH)2������
��3���ڶ��ι�����Ҫ���Ƚ��У���Ҫԭ����������������������Ҫ�ɷ����� ��
��4������Һ���л��MgSO4��7H2O�����ʵ���������Ϊ��������Һ���м����������ڹ��ˣ��ó�������������������Ũ�������½ᾧ���ݹ��ˡ�ϴ�ӵò�Ʒ��
��5������õ�MgSO4��7H2O����Ϊ24.6 g����������к�þ[��Mg(OH)2��]�İٷֺ���Լ ��MgSO4��7H2Oʽ��Ϊ246��

��֪��(��) Ksp[Mg(OH)2]��6.0��
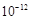
(��) Fe2+��Fe3+��Al3+��ʼ��������ȫ������pH��Χ����Ϊ��7.1~9.6��2.0~3.7��3.1~4.7
(��) ���ֻ�������ܽ�ȣ�S�����¶ȱ仯������ͼ��

�ش��������⣺
��1���������м�H2SO4��Һ����pHΪ1��2�Լ���һ����е�Ŀ���ǣ�������
��2������Һ��Mg2+��Ũ��Ϊ6 mol/L����ҺpH�������ſ��ܲ���Mg(OH)2������
��3���ڶ��ι�����Ҫ���Ƚ��У���Ҫԭ����������������������Ҫ�ɷ����� ��
��4������Һ���л��MgSO4��7H2O�����ʵ���������Ϊ��������Һ���м����������ڹ��ˣ��ó�������������������Ũ�������½ᾧ���ݹ��ˡ�ϴ�ӵò�Ʒ��
��5������õ�MgSO4��7H2O����Ϊ24.6 g����������к�þ[��Mg(OH)2��]�İٷֺ���Լ ��MgSO4��7H2Oʽ��Ϊ246��
��15�֣�
��1�����Mg2+�Ľ�ȡ�ʣ�2�֣�
��2��8��2�֣�
��3���¶Ƚϸ�ʱ������þ�η�������ף��������CaSO4��2H2O�ܽ��С����2�֣�
Al(OH)3��Fe(OH)3��CaSO4��2H2O ��2�֣�
��4��NaOH��Һ��2�֣� ������м�����ϡ���ᣨ2�֣�
��5��20.0%��3�֣�
��1�����Mg2+�Ľ�ȡ�ʣ�2�֣�
��2��8��2�֣�
��3���¶Ƚϸ�ʱ������þ�η�������ף��������CaSO4��2H2O�ܽ��С����2�֣�
Al(OH)3��Fe(OH)3��CaSO4��2H2O ��2�֣�
��4��NaOH��Һ��2�֣� ������м�����ϡ���ᣨ2�֣�
��5��20.0%��3�֣�
���������������ͼ���٢ڢ�������ܽ⣬�ܷ�����Һ�������ʣ���Ҫ��H2SiO3�Ͳ��ܽ���������ݽ�Fe2�� ������Fe3�� �Ա�ֲ���ȥ������Fe (OH)3��Al (OH)3��MgSO4��NaCl��Һ������Һ��õ���Ʒ����ͨ�������߷�Ӧ���ʣ����Mg2�� �Ľ����ʣ��𰸣����Mg2+�Ľ�ȡ�ʣ���Ksp[Mg(OH)2]��6.0��
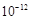 =c(Mg2��)c2(OH�D )�ã�c2(OH�D )= 6.0��
=c(Mg2��)c2(OH�D )�ã�c2(OH�D )= 6.0��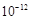 /6=1.0��10-12��c(OH�D )=1.0��10�D6mol/L,PH
/6=1.0��10-12��c(OH�D )=1.0��10�D6mol/L,PH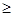 8���𰸣�8���Ǵ�ͼ�ж���������CaSO4��2H2O�ܽ��С������Һ��������ף��𰸣��¶Ƚϸ�ʱ������þ�η�������ף��������CaSO4��2H2O�ܽ��С���� Al(OH)3��Fe(OH)3��CaSO4��2H2O������Һ���к���MgSO4��NaCl��Ҫ��NaCl�����ȥ��Ҫ�Ƚ�Mg2�� �γ�Mg(OH)2���������˺�NaCl��ȥ��Ȼ���H2SO4��������MgSO4��Ȼ������Ũ�������½ᾧ�����ˡ�ϴ�ӡ�������þ��塣�𰸣�NaOH��Һ��������м�����ϡ�����MgSO4��7H2O����Ϊ24.6 g����0.1mol,��0.1molMg (OH)2Ϊ5.8 g��5.8g/29g��100%=20.0%���𰸣�20.0%��
8���𰸣�8���Ǵ�ͼ�ж���������CaSO4��2H2O�ܽ��С������Һ��������ף��𰸣��¶Ƚϸ�ʱ������þ�η�������ף��������CaSO4��2H2O�ܽ��С���� Al(OH)3��Fe(OH)3��CaSO4��2H2O������Һ���к���MgSO4��NaCl��Ҫ��NaCl�����ȥ��Ҫ�Ƚ�Mg2�� �γ�Mg(OH)2���������˺�NaCl��ȥ��Ȼ���H2SO4��������MgSO4��Ȼ������Ũ�������½ᾧ�����ˡ�ϴ�ӡ�������þ��塣�𰸣�NaOH��Һ��������м�����ϡ�����MgSO4��7H2O����Ϊ24.6 g����0.1mol,��0.1molMg (OH)2Ϊ5.8 g��5.8g/29g��100%=20.0%���𰸣�20.0%��
��ϰ��ϵ�д�
�����Ŀ
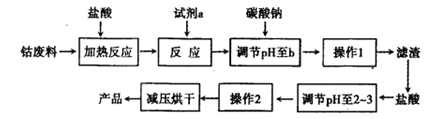
 I2��I������ʢ��KI3��Һ���Թ��м�������CCl4�����ú�CCl4������ɫ��˵��KI3��CCl4�е��ܽ�ȱ���ˮ�еĴ�
I2��I������ʢ��KI3��Һ���Թ��м�������CCl4�����ú�CCl4������ɫ��˵��KI3��CCl4�е��ܽ�ȱ���ˮ�еĴ�
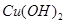 ����Һ
����Һ