��Ŀ����
��8�֣�X��Y��Z���ֶ�����Ԫ�أ����ǵ�ԭ������֮��Ϊ16��X��Y��Z����Ԫ�صij��������ڳ����¶�����ɫ���壬���ʵ������¿ɷ�����ͼ��ʾ�仯����֪һ��B�����к��е�ZԪ�ص�ԭ�Ӹ�����һ��C��������һ������ش��������⣺

��1��B���ӵĽṹʽ�� ��
��2��X������Z���ʿ��Ƴ����͵Ļ�ѧ��Դ��KOH��Һ���������Һ���������缫���ɶ�
����̿�Ƴɣ�ͨ��������ɿ�϶���ݳ����ڵ缫����ŵ磬������ͨ�� ����
���ƣ��������缫��ӦʽΪ ��
��3��C��һ�������·�Ӧ����A�Ļ�ѧ����ʽΪ ��

��1��B���ӵĽṹʽ�� ��
��2��X������Z���ʿ��Ƴ����͵Ļ�ѧ��Դ��KOH��Һ���������Һ���������缫���ɶ�
����̿�Ƴɣ�ͨ��������ɿ�϶���ݳ����ڵ缫����ŵ磬������ͨ�� ����
���ƣ��������缫��ӦʽΪ ��
��3��C��һ�������·�Ӧ����A�Ļ�ѧ����ʽΪ ��
��1����2�֣�H��O��H
(2)������1�֣���H2��2e��+2OH��=2H2O��3�֣�����ϵ���������ȿ�1 �֣�
(3)��2�֣�4NH3+5O2 4NO+6H2O����Ӧ����д�ɡ�һ�����������۷֣�
4NO+6H2O����Ӧ����д�ɡ�һ�����������۷֣�
(2)������1�֣���H2��2e��+2OH��=2H2O��3�֣�����ϵ���������ȿ�1 �֣�
(3)��2�֣�4NH3+5O2
 4NO+6H2O����Ӧ����д�ɡ�һ�����������۷֣�
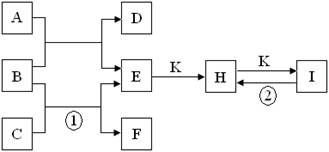
4NO+6H2O����Ӧ����д�ɡ�һ�����������۷֣�������������ͼ�⣬�������͵���Ŀ�ؼ�����ͻ�Ƶ㡣���������ڳ����¶�����ɫ����һ���������������͵�������H��N��O����Ԫ�ص�ԭ������ǡ������16������֮�仯�ϵ���������H2O��NO��NH3����Ϊһ��B�����к��е�ZԪ�ص�ԭ�Ӹ�����һ��C��������һ��������B��H2O��C��NH3��ZԪ������Ԫ�أ���X����Ԫ�أ�Y�ǵ�Ԫ�ء�
��1��B��ˮ������2��H��O��,�ṹʽΪH��O��H��
��2��������ȼ��ʱʧȥ���ӣ�����������������Ӧ��������ȼ�ϵ��������ͨ������������ͨ�������������缫��ӦʽΪH2��2e��+2OH��=2H2O��
��3�������е�Ԫ���ǣ�3�ۣ�������ͻ��ϼۣ����Ա���������NO������ʽΪ4NH3+5O2 4NO+6H2O
4NO+6H2O
��1��B��ˮ������2��H��O��,�ṹʽΪH��O��H��
��2��������ȼ��ʱʧȥ���ӣ�����������������Ӧ��������ȼ�ϵ��������ͨ������������ͨ�������������缫��ӦʽΪH2��2e��+2OH��=2H2O��
��3�������е�Ԫ���ǣ�3�ۣ�������ͻ��ϼۣ����Ա���������NO������ʽΪ4NH3+5O2
 4NO+6H2O
4NO+6H2O
��ϰ��ϵ�д�
�����Ŀ
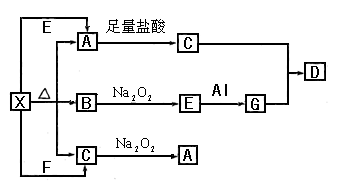
 A�����ӷ���ʽ��
A�����ӷ���ʽ�� 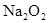 �μӷ�Ӧ�Ļ�ѧ����ʽ______________________________________����0.2mol
�μӷ�Ӧ�Ļ�ѧ����ʽ______________________________________����0.2mol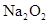 �μӷ�Ӧ,��ת�Ƶĵ�����Ϊ_____________����
�μӷ�Ӧ,��ת�Ƶĵ�����Ϊ_____________����