题目内容
【题目】I、实验室中常用MnO2氧化浓盐酸的方法制取氯气,实验装置如图所示:
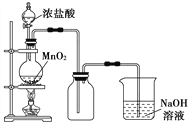
(1)圆底烧瓶中发生反应的化学反应方程式是__________________。
(2)如果将过量二氧化锰与20 mL 12 mol·L-1的盐酸混合加热(忽略盐酸的挥发),充分反应后生成的氯气明显_________(填大于、等于、小于)0.06 mol。其主要原因有_____________________________;
(3)写出尾气处理的离子方程式是_______________________。
II、用Na2CO3·10H2O晶体,配制0.2 mol·L-1的Na2CO3溶液480 mL。
(1)应称取Na2CO3·10H2O晶体的质量:____________。定容时,向容量瓶中加水,至1~2cm时,改用_________加水至刻度,加盖摇匀;
(2)下列操作对所配溶液的浓度可能产生影响
①Na2CO3·10H2O晶体失去了部分结晶水 ②用“左码右物”的称量方法称量晶体(使用游码) ③碳酸钠晶体不纯,其中混有氯化钠 ④容量瓶未经干燥使用。 其中引起所配溶液浓度偏高的有______________(填序号)。
【答案】MnO2+4HCl( 浓)![]() MnCl2+Cl2↑+2H2O小于随反应的进行,盐酸浓度变小,稀盐酸和二氧化锰不反应Cl2+2OH–=Cl–+ClO–+H2O28.6g胶头滴管①
MnCl2+Cl2↑+2H2O小于随反应的进行,盐酸浓度变小,稀盐酸和二氧化锰不反应Cl2+2OH–=Cl–+ClO–+H2O28.6g胶头滴管①
【解析】试题分析:I、本题考查实验室制取氯气。(1)固体二氧化锰和浓盐酸加热反应得到氯气;(2)二氧化锰与浓盐酸反应生成氯气,二氧化锰与稀盐酸不反应;(3)氯气能溶于氢氧化钠溶液;II、本题考查用固体配制一定物质的量浓度的溶液的实验步骤、配制480mL溶液需要500mL的容量瓶;误差分析依据C(测)V(测)=C(标)V(标)分析;
解析:(1)圆底烧瓶中二氧化锰和浓盐酸发生反应生成氯气的反应方程式是MnO2+4HCl( 浓)![]() MnCl2+Cl2↑+2H2O ;(2) 二氧化锰与浓盐酸反应生成氯气,二氧化锰与稀盐酸不反应,将过量二氧化锰与20 mL 12 mol·L-1的盐酸混合加热(忽略盐酸的挥发),盐酸变稀后不再反应,最终盐酸有剩余,所以充分反应后生成的氯气明显小于0.06 mol。(3)用氢氧化钠吸收氯气生成氯化钠、次氯酸钠和水,离子方程式是Cl2+2OH– =Cl–+ClO–+H2O;
MnCl2+Cl2↑+2H2O ;(2) 二氧化锰与浓盐酸反应生成氯气,二氧化锰与稀盐酸不反应,将过量二氧化锰与20 mL 12 mol·L-1的盐酸混合加热(忽略盐酸的挥发),盐酸变稀后不再反应,最终盐酸有剩余,所以充分反应后生成的氯气明显小于0.06 mol。(3)用氢氧化钠吸收氯气生成氯化钠、次氯酸钠和水,离子方程式是Cl2+2OH– =Cl–+ClO–+H2O;
II、用Na2CO3·10H2O晶体,配制0.2 mol·L-1的Na2CO3溶液480 mL。
(1)配制0.2 mol·L-1的Na2CO3溶液480 mL,需要500mL的容量瓶,应称取Na2CO3·10H2O晶体的质量:0.2 mol·L-1×0.5L×286g/mol=28.6g。定容时,向容量瓶中加水,至1~2cm时,改用胶头滴管加水至刻度,加盖摇匀;
(2)①Na2CO3·10H2O晶体失去了部分结晶水,碳酸钠质量偏大,所得溶液浓度偏高; ②用“左码右物”的称量方法称量晶体(使用游码),晶体质量小于28.6g,所得溶液浓度偏小; ③碳酸钠晶体不纯,其中混有氯化钠,碳酸钠质量偏小,所得溶液浓度偏小; ④容量瓶未经干燥使用,无影响。 所以 其中引起所配溶液浓度偏高的有①。

 ABC考王全优卷系列答案
ABC考王全优卷系列答案
