��Ŀ����
8��CO�����Ǽ���ú������Ҫ�ɷ֣�Ҳ����Ҫ�Ļ���ԭ�ϣ���ͼ��ijЩ���л����ڵ��¡���ѹ�ʹ��������£��ϳɾ����������ܵ�װ���Ը߷���Ϳ�ϡ�ճ�����Ļ������̣�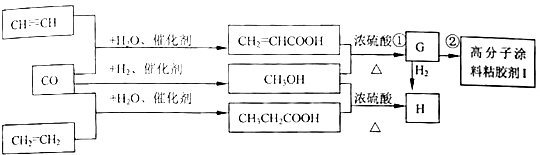
�ش��������⣺
��1��д���������ʵĽṹ��ʽ��GCH2=CHCOOCH3��HCH3CH2COOCH3��I
 ��
����2��д��ͼ�Т٢ڵķ�Ӧ���ͣ���������Ӧ���ڼӾ۷�Ӧ��
��3��д��ͼ������CH3OH���״����Ļ�ѧ����ʽCO+2H2$\stackrel{����}{��}$CH3OH���ɼ״�����H�Ļ�ѧ����ʽCH3CH2CO0H+HOCH3$?_{��}^{Ũ����}$CH3CH2COOCH3+H2O��
���� ����CH2=CHCO0H��HOCH3����������Ӧ���ɵ�GΪCH2=CHCOOCH3��CH2=CHCOOCH3�к���̼̼˫�����ܹ������Ӿ۷�Ӧ�����ɵ�IΪ ��CH3CH2CO0H��HOCH3����������Ӧ���ɵ�HΪCH3CH2COOCH3���ݴ˷�����
��CH3CH2CO0H��HOCH3����������Ӧ���ɵ�HΪCH3CH2COOCH3���ݴ˷�����
��� �⣺��1������CH2=CHCO0H��HOCH3����������Ӧ���ɵ�GΪCH2=CHCOOCH3��CH2=CHCOOCH3�к���̼̼˫�����ܹ������Ӿ۷�Ӧ�����ɵ�IΪ ��CH3CH2CO0H��HOCH3����������Ӧ���ɵ�HΪCH3CH2COOCH3���ʴ�Ϊ��CH2=CHCOOCH3��CH3CH2COOCH3��
��CH3CH2CO0H��HOCH3����������Ӧ���ɵ�HΪCH3CH2COOCH3���ʴ�Ϊ��CH2=CHCOOCH3��CH3CH2COOCH3�� ��
��
��2��CH2=CHCO0H��HOCH3����������Ӧ���ɵ�GΪCH2=CHCOOCH3��CH2=CHCOOCH3�к���̼̼˫�����ܹ������Ӿ۷�Ӧ�����ɵ�IΪ ���ʴ�Ϊ��������Ӧ���Ӿ۷�Ӧ��
���ʴ�Ϊ��������Ӧ���Ӿ۷�Ӧ��
��3��CO��H2�ڴ����������ܹ����ɼ״�����ѧ����ʽΪCO+2H2$\stackrel{����}{��}$CH3OH��CH3CH2CO0H��HOCH3����������Ӧ���ɵ�HΪCH3CH2COOCH3����ѧ����ʽΪCH3CH2CO0H+HOCH3$?_{��}^{Ũ����}$CH3CH2COOCH3+H2O���ʴ�Ϊ��CO+2H2$\stackrel{����}{��}$CH3OH��CH3CH2CO0H+HOCH3$?_{��}^{Ũ����}$CH3CH2COOCH3+H2O��
���� ���⿼���л�����ƶ���ϳɣ�Ϊ��Ƶ���㣬������ѧ���ķ��������Ŀ��飬ע������л���Ľṹ���з����жϣ��Ƕ�ѧ���ۺ������������������Ŀ��飬����ʱע��������ʵ����ʣ�

 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�| A�� | Ư��Ϊ������ | B�� | ���άΪ������ | ||
| C�� | ������Ϊ���������� | D�� | ������Һ���ڽ��� |
| A�� | �������ƹ�����ˮ��Ӧ��2O22-+2H2O�T4OH-+O2�� | |
| B�� | ������[KAl��SO4��2]��Һ����μ���Ba��OH��2��Һ��SO42-ǡ�ó�����ȫ��2Al3++3SO42-+3Ba2++6OH-�T2Al��OH��3��+3BaSO4�� | |
| C�� | FeSO4������Һ��¶�ڿ����У�4Fe2++O2+4H+�T4Fe3++2H2O | |
| D�� | �����������Һ��ͨ������SO2��ClO-+SO2+H2O�TCl-+SO42-+H+ |
| A�� | 6+m | B�� | 6-m | C�� | 3+m | D�� | 3-m |
| ʵ����� | �� | �� | �� |
| �Ͻ�����g | 0.255 | 0.385 | 0.459 |
| �����������/mL | 280 | 336 | 336 |
��1������һ���������ǣ�����ţ��ף�����һ�������������Һͱ���
��2��Ҫ����Ͻ���þ���������������ṩ�����������������ݵ��Ǽף�þ�ںϽ��е���������Ϊ53.3%��
��3���ڱ���ʵ��֮���������м���1mol/L�Ŀ�������Һ����ǡ��ʹ��Ԫ��ȫ����ƫ�������ʽ���ڣ���ʹþ����
�պó�����ȫ������Һ��ƫ�����Ƶ����ʵ���Ϊ0.009mol��������Ŀ�������Һ�����Ϊ39mL��
| A�� | ��ȡ | B�� | �ᾧ | C�� | ���� | D�� | ���� |

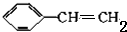 ��
��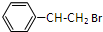 ��
��