��Ŀ����
13���ڱ�״���½��мס��ҡ�������ʵ�飮��ȡ30mLͬŨ�ȵ�������Һ�����벻ͬ������ͬһ��þ���Ͻ��ĩ���������壮�й����ݼ�¼�����| ʵ����� | �� | �� | �� |
| �Ͻ�����g | 0.255 | 0.385 | 0.459 |
| �����������/mL | 280 | 336 | 336 |
��1������һ���������ǣ�����ţ��ף�����һ�������������Һͱ���
��2��Ҫ����Ͻ���þ���������������ṩ�����������������ݵ��Ǽף�þ�ںϽ��е���������Ϊ53.3%��
��3���ڱ���ʵ��֮���������м���1mol/L�Ŀ�������Һ����ǡ��ʹ��Ԫ��ȫ����ƫ�������ʽ���ڣ���ʹþ����
�պó�����ȫ������Һ��ƫ�����Ƶ����ʵ���Ϊ0.009mol��������Ŀ�������Һ�����Ϊ39mL��
���� ��1������Ũ�ȡ����һ�������кϽ�����С�����кϽ��������Ҽ��������������С���������������˵���������������������ȫ��Ӧ�����кϽ�����С�ڱ��кϽ����������ҡ����������������ȣ�˵���ҡ�����������ȫ��Ӧ�����ݼ��н�����������������ȹ�ϵ��������336mL������Ҫ������������ȷ����������������Ƿ�ǡ�÷�Ӧ��
��2������������ʣ�࣬������ȫ��Ӧ����ʱ��������280mL���ʿ��Ը��ݼ������ݼ�����������ʵ�������þ���������ʵ����ֱ�Ϊxmol��ymol�����ݶ�������֮�������ת���غ��з��̼���x��y��ֵ���ٸ���m=nM�����þ����������������Ͻ���þ������������
��3��ʵ��֮���������м���NaOH��Һ��ǡ��ʹ�Ͻ��е���Ԫ��ȫ��ת��ΪAlO2-����ʹMg2+�պó�����ȫ����Ӧ����Һ������Ϊ�Ȼ��ơ�ƫ�����ƣ��ɣ�2���м���Mg��Al�����ʵ�����֪����Al�����ʵ�����������Ԫ���غ����ƫ�����Ƶ����ʵ����������������غ������Һ��n��NaCl��=n��HCl���������������غ��֪n��NaOH��=n��NaCl��+n��NaAlO2����n��Na+��=n��NaOH�����ٸ���V=$\frac{n}{c}$�����������Ƶ������
��� �⣺��1�����ݱ������ݿ�֪������Ũ�ȡ����һ�������кϽ�����С�����кϽ��������Ҽ��������������С���������������˵���������������������ȫ��Ӧ�����кϽ�����С�ڱ��кϽ����������ҡ����������������ȣ�˵���ҡ�����������ȫ��Ӧ������336mL������Ҫ����������Ϊ0.255g��$\frac{336mL}{280mL}$=0.306g�����ҡ����н���ʣ�࣬����㣬
�ʴ�Ϊ���ף��Һͱ���
��2������������ʣ�࣬������ȫ��Ӧ����ʱ��������280mL���ʿ��Ը��ݼ������ݼ�����������ʵ���֮�ȣ���þ���������ʵ����ֱ�Ϊxmol��ymol�����ݶ���������֪24x+27y=0.255�����ݵ���ת���غ���2x+3y=$\frac{0.28L}{22.4L/mol}$��2��������ã�x=0.005��y=0.005��
�ʺϽ���þ����������Ϊ��$\frac{24g/mol��0.005mol}{0.255g}$��100%��53.3%��
�ʴ�Ϊ��53.3%
��3����ʵ��֮���������м���NaOH��Һ��ǡ��ʹ�Ͻ��е���Ԫ��ȫ��ת��ΪAlO2-����ʹMg2+�պó�����ȫ����Ӧ����Һ������Ϊ�Ȼ��ơ�ƫ�����ƣ��ɣ�2���м���Mg��Al�����ʵ�����֪����Al�����ʵ���Ϊ��0.005mol��$\frac{0.459g}{0.255g}$=0.009mo��������Ԫ���غ��֪��n��NaAlO2��=n��Alԭ�ӣ�=0.009mol�������������غ��֪��n��NaCl��=1mol/L��0.03L=0.03mol�������������غ��֪��n��NaOH��=n��NaCl��+n��NaAlO2��=0.03mol+0.009mol=0.039mol��n��Na+��=n��NaOH��=0.039mol��
����Ҫ����������Һ�����Ϊ��$\frac{0.039mol}{1mol/L}$=0.039L=39mL��
�ʴ�Ϊ��0.009��39��
���� ���⿼������ļ��㣬��Ŀ�Ѷ��еȣ����ݱ������ݹ�ϵ�жϷ�Ӧ�Ĺ��������ǹؼ�����3��ע�������غ�˼����������ؿ���ѧ���ķ�����������������ѧ����������

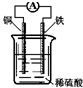
| A�� | ��װ���ܽ�����ת��Ϊ��ѧ�� | B�� | ͭ�����ܽ� | ||
| C�� | ����������ͨ����������ͭ�� | D�� | ������ӦΪFe-2e-�TFe2+ |
| A�� | �����ܷ��ǰ��շ�Ӧ��ԭ����Ƶ� | |
| B�� | �����ܷ��ǰ��շ�Ӧ��ԭ����Ƶ� | |
| C�� | ��Ӧ�٢ھ�������Ƴɼ����ܷ��������ǵ���ͬ��KIO3������SO2�٣����Լ����ܷ��ǰ�����Ƶ� | |
| D�� | ��Ӧ�٢ھ�������Ƴɼ����ܷ��������ǵ���ͬ��KIO3������SO2�࣬���Լ����ܷ��ǰ�����Ƶ� |
| A�� | ������Ȼ�̼ | B�� | AgCl��ˮ | ||
| C�� | һ����̼�Ͷ�����̼ | D�� | ���ۺ�ͭ�� |
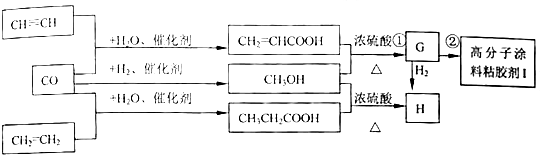
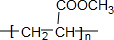 ��
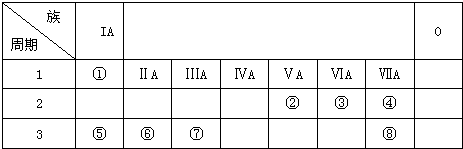
��
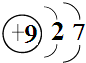 ��
��