��Ŀ����
��֪����������Ԫ��X��Y��Z��W��ԭ����������������X��Y��ԭ������֮�͵���Z��ԭ��������X��Z���γ�X2Z��X2Z2���ֻ����W�Ƕ���������Ԫ���а뾶����Ԫ�أ�
(1)W�����ڱ��е�λ�ã�________��
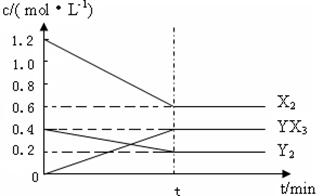
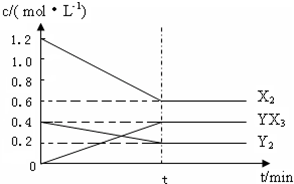
(2)��һ�������£��ݻ�Ϊ1 L�ܱ������м���1.2 mol��X2��0.4 mol��Y2���������·�Ӧ��3X2(g)��Y2(g)![]() 2YX3(g)����H��Ӧ�����ʵ���Ũ����ʱ��仯���£�
2YX3(g)����H��Ӧ�����ʵ���Ũ����ʱ��仯���£�

�ٴ˷�Ӧ��ƽ�ⳣ������ʽΪ________(�û�ѧʽ��ʾ)��K��________��
���������¶�ƽ�ⳣ��K��С����H________0(�����)��
(3)A1������Ԫ��������Ԫ����ɵĵ���ʣ���Һ�ʼ��ԣ���0.1 mol��L��1��A1��Һϡ����ԭ�����10������Һ��pH��12����A1�ĵ���ʽΪ________��
(4)B1��B2��������Ԫ�������γɵ�ǿ����ʣ�����Һ�����ԣ���ͬŨ��ʱB1��Һ��ˮ�ĵ���̶�С��B2��Һ��ˮ�ĵ���̶ȣ���ԭ����________��
(5)A2��B1��Ӧ����B2����0.2 mol/L��A2��0.1 mol/L��B1�������Ϻ���Һ������Ũ�ȴ�С��ϵΪ________��
�𰸣�

��ϰ��ϵ�д�
�����Ŀ
��֪����������Ԫ��X��Y��Z��W��Q��ԭ����������������X��Z��Y��W�ֱ�Ϊͬ����Ԫ�أ�XԪ�ص�ԭ���ڲ��������������������һ�룬W������������Ӧ��ˮ�����Ƕ�Ԫǿ�ᣬ������˵������ȷ����
��������
��������
| A������Ԫ�ؾ����γɲ�ͬ��ͬ�������� | B��X��Y��W�ֱ�����γ�XY2��XW2������ | C���⻯���ȶ�����ǿ�������ǣ�Q��W��Z��X | D��W�γɼ������Ӱ뾶С��Q�γɼ������Ӱ뾶 |
 2YX3(g) ��H
2YX3(g) ��H 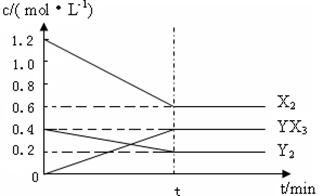
 2YX3(g) ��H
2YX3(g) ��H