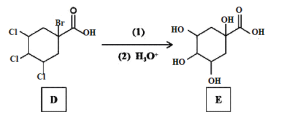
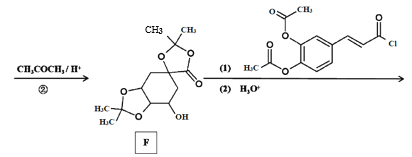
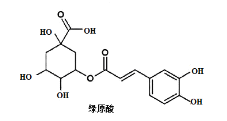
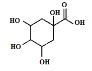
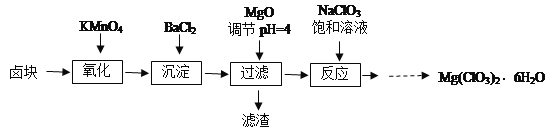
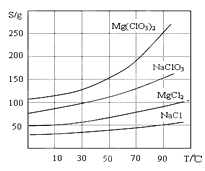
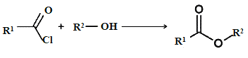��Ŀ����
����Ŀ��һ��Ru�������g��C3N4���Ϲ������CO2��ԭΪHCOOH��ԭ��ʾ��ͼ��ͼ��
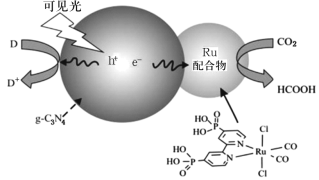
��1��Ru��̬ԭ�Ӽ۵����Ų�ʽΪ4d75s1��д����Ԫ����Ԫ�����ڱ��е�λ��___������___����
��2��HCOOH����������������Ŀ֮����___��HCOOH�ķе��CO2�ߵ�ԭ��___��
��3�������Ĺ��������е�����ԼΪ399kJ��mol1�������±��йص����ʷ�������Ҫ��ѧ������Ϣ��˵�����峤ʱ������������Ƥ�������˺���ԭ��__��
��4����֪![]() ��
��![]() ������ԭ�Ӿ����棬���е�ԭ�ӽ����γ���λ������___������ǰ������������������
������ԭ�Ӿ����棬���е�ԭ�ӽ����γ���λ������___������ǰ������������������
��5������״̬�ĵ�����ԭ���У����������һ��������������������___������ţ���ͬ������С����___(�����)��
A.��![]()
B.��![]()
C.��![]()
D.��![]()
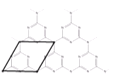
��6��һ����ʯī�ľۺ���뵼��g��C3N4���䵥��ƽ��ṹ��ͼ1�������ṹ��ͼ2��

��g��C3N4�е�ԭ�ӵ��ӻ�������__��
�ڸ���ͼ2����ͼ1����ƽ���ı��λ���һ����С�ظ���Ԫ___��
����֪�þ��������ΪVcm3���м��ԭ�Ӿ��ھ����ڲ����谢���ӵ�������ֵΪNA����g��C3N4���ܶ�Ϊ__g��cm-3��
���𰸡������������� d 4��1 HCOOH���Ӽ����γ���� �����Ĺ��������е������ȵ����ʷ����еĻ�ѧ�����ܴ�ʱ������ʹ��ѧ�����ѣ��Ӷ��ƻ������ʷ��� ���� C D sp2�ӻ�  ��
��
![]()
��������
��1����Ru��̬ԭ�Ӽ۵����Ų�ʽΪ4d75s1��֪��RuԪ��λ�����ڱ������������壬����d����
��2��̼ԭ���γɵĵ���ȫ���������γɵ�˫����һ����������һ����������HCOOH�����к���4��������1��������HCOOH��CO2��Ϊ���Ӿ��壬HCOOH�����к����Ȼ������Ӽ����γ��������������̼���Ӽ䲻���γ������
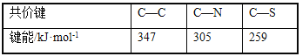
��3�������Ĺ��������е�����ԼΪ399kJ/mol���ȵ����ʷ�����C-C��C-N��C-S�ļ��ܶ���
��4��![]() ��Nԭ�Ӳ����γɴ����������������Ӷ��γ���λ����
��Nԭ�Ӳ����γɴ����������������Ӷ��γ���λ����![]() ��Nԭ����һ�Թµ��ӣ����������Ӷ��γ���λ����
��Nԭ����һ�Թµ��ӣ����������Ӷ��γ���λ����
(5)ͬһԪ�ص�ԭ�ӣ����������һ��������������������̬ԭ��<��̬�ĵ�һ������<�ڶ������ܣ����ܼ�����ȫ����������ȫ��ʱ�����ȶ������������һ��������Ҫ�������ߣ�
(6)�ٸ�������Nԭ�Ӽ۲���Ӷ���Ϊ3��
����ͼ2��֪���ظ��Ľṹ��ԪΪ��Ԫ�����������Nԭ���γɵĽṹ��
���ɾ�̯������ɵá�
��1����Ru��̬ԭ�Ӽ۵����Ų�ʽΪ4d75s1��֪��RuԪ��λ�����ڱ������������壬����d�����ʴ�Ϊ�������������壬d��
��2��̼ԭ���γɵĵ���ȫ���������γɵ�˫����һ����������һ����������HCOOH�����к���4��������1����������HCOOH��������������������Ŀ֮����4:1��HCOOH��CO2��Ϊ���Ӿ��壬HCOOH�����к����Ȼ������Ӽ����γ��������������̼���Ӽ䲻���γ��������HCOOH�ķе��CO2�ߣ��ʴ�Ϊ��4:1��HCOOH���Ӽ����γ������
��3�������Ĺ��������е�����ԼΪ399kJ/mol���ȵ����ʷ�����C-C��C-N��C-S�ļ��ܶ���ʱ�������������ʹ�����ʷ����еĻ�ѧ�����ѣ��Ӷ��ƻ������ʷ��ӣ��ʴ�Ϊ�������Ĺ��������е������ȵ����ʷ����еĻ�ѧ�����ܴ�ʱ������ʹ��ѧ�����ѣ��Ӷ��ƻ������ʷ��ӣ�
��4��![]() ��Nԭ�Ӳ����γɴ����������������Ӷ��γ���λ������
��Nԭ�Ӳ����γɴ����������������Ӷ��γ���λ������![]() ��Nԭ����һ�Թµ��ӣ����������Ӷ��γ���λ��������߷����е�ԭ�ӽ����γ���λ�����ʴ�Ϊ�����ߣ�
��Nԭ����һ�Թµ��ӣ����������Ӷ��γ���λ��������߷����е�ԭ�ӽ����γ���λ�����ʴ�Ϊ�����ߣ�
��5�����ĽṹԽ�ȶ������������һ��������������Խ�ߣ�
A. ![]() Ϊ��̬Nԭ�ӵĹ����ʾʽ��2p�ܼ�����������ȶ���
Ϊ��̬Nԭ�ӵĹ����ʾʽ��2p�ܼ�����������ȶ���
B. ![]() Ϊ��̬Oԭ�ӵĹ����ʾʽ��ʧȥ1������ΪO�ĵ�һ�����ܣ�
Ϊ��̬Oԭ�ӵĹ����ʾʽ��ʧȥ1������ΪO�ĵ�һ�����ܣ�
C. ![]() ʧȥ�����һ������ΪO�ĵڶ������ܣ�
ʧȥ�����һ������ΪO�ĵڶ������ܣ�
D. ![]() Ϊ����̬Oԭ�ӣ�
Ϊ����̬Oԭ�ӣ�
ͬһԪ�ص�ԭ�ӣ����������һ��������������������̬ԭ��<��̬�ĵ�һ������<�ڶ������ܣ����ܼ�����ȫ����������ȫ��ʱ�����ȶ������������һ��������Ҫ�������ߣ�����������һ��������������������C����С����D���ʴ�Ϊ��C��D��
��6���ٸ�������Nԭ�Ӽ۲���Ӷ���Ϊ3�����ݼ۲���ӶԻ��������ж�Nԭ���ӻ�����Ϊsp2�ӻ����ʴ�Ϊ��sp2�ӻ���
����ͼ2��֪���ظ��Ľṹ��ԪΪ��Ԫ�����������Nԭ���γɵĽṹ����С�ظ���Ԫ��ͼ��ʾ�� ��
�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
��
���ɾ�̯����֪���þ�����Nԭ�Ӹ���=8��![]() +8��
+8��![]() +2��
+2��![]() +4=8��Cԭ�Ӹ���=3+6��
+4=8��Cԭ�Ӹ���=3+6��![]() =6��1������������=
=6��1������������=![]() ���ܶ���=
���ܶ���=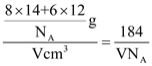 g.cm-3���ʴ�Ϊ��
g.cm-3���ʴ�Ϊ��![]() ��
��

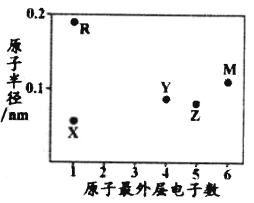
����Ŀ��������Ԫ��A��B��D��E��G��J�����ڱ��е�λ�����£�
A | |||||||
B | D | ||||||
E | G | J |
�����ϱ��ش����⣺
(1)![]() �����ڱ��е�λ����______��D��ԭ�ӽṹʾ��ͼ_____��
�����ڱ��е�λ����______��D��ԭ�ӽṹʾ��ͼ_____��
(2)![]() ��B��E��G��ԭ�Ӱ뾶�ɴ�С��˳����_____
��B��E��G��ԭ�Ӱ뾶�ɴ�С��˳����_____![]() ��Ԫ�ط���
��Ԫ�ط���![]() ��
��
(3)![]() ��D����̬�⻯����ȶ��Թ�ϵΪ______
��D����̬�⻯����ȶ��Թ�ϵΪ______![]() �ѧʽ
�ѧʽ![]() �����Ƕ�����______
�����Ƕ�����______![]() ���ӻ�����ۻ�����
���ӻ�����ۻ�����![]() ��
��
(4)��������Ԫ�ص�����������Ӧ��ˮ�����У���һ�����ʼ�����ǿ�ᷴӦ������ǿ�Ӧ��д���������ʸ�����������Һ��Ӧ�Ļ�ѧ����ʽ________________��