��Ŀ����
10����֪����C12ͨ������NaOH��Һ�������п�����NaCl��NaClO��NaClO3����$\frac{c��C{l}^{-}��}{c��Cl{O}^{-}��}$��ֵ�����¶ȸߵ��йأ���n��NaOH��=6amolʱ�������й�˵����ȷ���ǣ�������| A�� | �ı��¶ȣ���Ӧ��ת�Ƶ��ӵ����ʵ���n�ķ�Χ��3amol��n��5amol | |
| B�� | �ı��¶ȣ�������NaCl����С���۲���Ϊ4amol | |
| C�� | �μӷ�Ӧ�����������ʵ���3amol | |
| D�� | ij�¶��£�����Ӧ��$\frac{c��C{l}^{-}��}{c��Cl{O}^{-}��}$=6������Һ��$\frac{c��C{l}^{-}��}{c��C{O}_{3}^{-}��}=\frac{1}{2}$ |
���� A����������ֻ��NaClO3ʱ��ת�Ƶ�����࣬��������ֻ��NaClOʱ��ת�Ƶ������٣����ݵ���ת���غ㼰�������غ���㣻
B����Ӧ��ת�Ƶ�������ʱ����NaCl���٣�
C����Clԭ���غ��֪��2n��Cl2��=n��NaCl��+n��NaClO��+n��NaClO3�������������غ��֪n��NaCl��+n��NaClO��+n��NaClO3��=n��NaOH����
D����n��ClO-��=1mol����Ӧ��$\frac{c��C{l}^{-}��}{c��Cl{O}^{-}��}$=6����n��Cl-��=6mol�����ݵ���ת���غ����n��ClO3-�����ݴ˼����жϣ�
��� �⣺A����������ֻ��NaClO3ʱ��ת�Ƶ�����࣬���ݵ���ת���غ�n��NaCl��=5��NaClO3�������������غ㣺n��NaCl��+n��NaClO3��=n��NaOH������n��NaClO3��=$\frac{1}{6}$n��NaOH��=$\frac{1}{6}$��6a mol��ת�Ƶ���������ʵ���=a mol��5=5a mol����������ֻ��NaClOʱ��ת�Ƶ������٣����ݵ���ת���غ�n��NaCl��=n��NaClO�������������غ㣺n��NaCl��+n��NaClO��=n��NaOH������n��NaClO��=$\frac{1}{2}$n��NaOH��=3a mol��ת�Ƶ�����С���ʵ���=3a mol��1=3a mol���ʷ�Ӧ��ת�Ƶ��ӵ����ʵ���n�ķ�Χ��3amol��n��5amol����A��ȷ��
B����Ӧ�л�ԭ����ֻ��NaCl����Ӧ��ת�Ƶ�������ʱ����NaCl���٣����ݵ���ת���غ�n��NaCl��=n��NaClO�������������غ㣺n��NaCl��+n��NaClO��=n��NaOH������n��NaCl��=$\frac{1}{2}$n��NaOH��=3a mol����B����
C����Clԭ���غ��֪��2n��Cl2��=n��NaCl��+n��NaClO��+n��NaClO3�������������غ��֪n��NaCl��+n��NaClO��+n��NaClO3��=n��NaOH�����ʲμӷ�Ӧ�����������ʵ���=$\frac{1}{2}$n��NaOH��=3a mol����C��ȷ��
D����n��ClO-��=1mol����Ӧ��$\frac{c��C{l}^{-}��}{c��Cl{O}^{-}��}$=6����n��Cl-��=6mol������ת���غ㣬5��n��ClO3-��+1��n��ClO-��=1��n��Cl-������5��n��ClO3-��+1��1mol=1��6mol�����n��ClO3-��=1mol������Һ��$\frac{c��C{l}^{-}��}{c��Cl{{O}_{3}}^{-}��}$=6����D����
��ѡAC��
���� ���⿼����������ԭ��Ӧ���㣬��Ŀ�Ѷ��еȣ�ע�����ת���غ㼰������Ӧ�ã������ڿ���ѧ���ķ��������ͼ���������

 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�| A�� | ���ִ�����Һ�����ʵ���Ũ�ȷֱ�Ϊc1��c2��pH�ֱ�Ϊa�ͣ�a+1��������c1=10c2�� | |
| B�� | 10mL0.1mol•L-1CH3COOH��Һ��������ʵ�����NaOH����Һ����c��Na+����c��CH3COO-����c��H+����c��OH-�� | |
| C�� | ��0.1mol•L-1NaHSO3��Һ����c��Na+��=c��HSO3-��+c��SO32-��+c��H2SO3�� | |
| D�� | pH��7��ij��Ԫ��������ʽ��NaHA��Һ����c��H+��+2c��A2-��=c��OH-��+c��H2A�� |
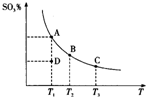 ��������Ĵ�����ԭ��Ϊ2SO2��g��+O2��g��?2SO3��g������Ӧ�����ϵ��ƽ��״̬ʱSO3�İٷֺ������¶ȵĹ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
��������Ĵ�����ԭ��Ϊ2SO2��g��+O2��g��?2SO3��g������Ӧ�����ϵ��ƽ��״̬ʱSO3�İٷֺ������¶ȵĹ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ��D��ʱ����Ӧ������� | |
| B�� | ��Ӧ2SO2��g��+O2��g��?2SO3��g���ġ�H��0 | |
| C�� | ��B��C���ƽ�ⳣ���ֱ�ΪKB��KC����KB��KC | |
| D�� | ���º�ѹ����ƽ����ϵ��ͨ�뺤����ƽ�������ƶ� |
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | �� | �� | �� | |
| 4 | �� |
��2���ۺ͢��������������Ӧ��ˮ����֮����Է�Ӧ��д����Ӧ�����ӷ���ʽAl��OH��3+OH-=AlO2-+2H2O��
��3���١��ޡ��ߡ����������������Ӧ��ˮ�������������Բ�ͬ����H2SiO3�����ѧʽ��
| A�� | 2K+�еġ�2����ʾ�����ӵĸ�����2 | |
| B�� | 3CO2�С�2����ʾÿ��������̼�����к���2����ԭ�� | |
| C�� | Cu2+�еġ�2����ʾͭ�Ļ��ϼ���+2�� | |
| D�� | H2O�еġ�2����ʾһ��ˮ�����к��е���ԭ������2 |
| A�� | һ��û�ж�����̼ | B�� | ���ܺ��м�������� | ||
| C�� | ����ֻ�м��� | D�� | ���ܺ���������һ����̼ |
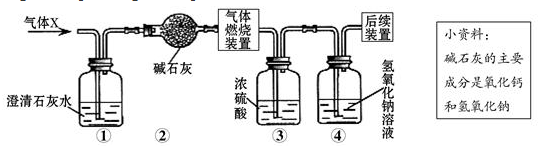
 ����ͼ��ʾװ�ý�������ˮ�ķ�Ӧʵ�飬��ش��������⣺
����ͼ��ʾװ�ý�������ˮ�ķ�Ӧʵ�飬��ش��������⣺