ЬтФПФкШн
ЁОЬтФПЁПtЁцЪБ,Яђ20.00mL0.1mol/LЖўдЊШѕЫсH2XШмвКжаЕЮШы0.1mol/LNaOHШмвК,ШмвКжагЩЫЎЕчРыГіЕФcЫЎ(OH-)ЕФИКЖдЪ§[-lgc ЫЎ(OH-)]гыЫљМгNaOHШмвКЬхЛ§ЕФЙиЯЕШчЭМЫљЪОЁЃЯТСаЫЕЗЈжаВЛе§ШЗЕФЪЧ
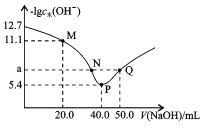
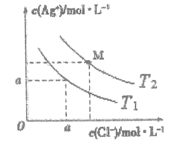
A.MЕуШмвКжа:c(Na+)>c(HX-)>c(H2X)
B.PЕуШмвКжа: c(OH-)-c(H+)=c(HX-)+2c(H2X)
C.ШмвКжа![]()
D.ЫЎЕФЕчРыГЬЖШ:P>N=Q>M,Чвa=7
ЁОД№АИЁПD
ЁОНтЮіЁП
ЖўдЊШѕЫсH2XШмвКжаЕЮNaOHШмвКЃЌЯШЗЂЩњH2X+OH-=H2O+HX-ЃЌШЛКѓЗЂЩњHX-+OH-=H2O+X2-ЃЛ
AЃЎMЕуМгШы20.0mLNaOHШмвКЃЌШмвКжаЕФШмжЪЮЊNaHXЃЌШмвКжаДцдкHX-ЕФЕчРыКЭЫЎНтЃЌЫљвдДЫЪБc(Na+)>c(HX-)ЃЌЫЎНтЪЧЮЂШѕЕФЫљвдc(HX-)>c(H2X)ЃЌЙЪAе§ШЗЃЛ
BЃЎPЕуМгШы40.0mLNaOHШмвКЃЌЫљвдШмжЪЮЊNa2XЃЌШмвКжаДцдкжЪзгЪиКуЃЌcЃЈOH-ЃЉ=cЃЈH+ЃЉ+cЃЈHX-ЃЉ+2cЃЈH2XЃЉЃЌМДc(OH-)-c(H+)=c(HX-)+2c(H2X)ЃЌЙЪBе§ШЗЃЛ
CЃЎ![]() ЃЌОнЭМПЩжЊЫцзХЧтбѕЛЏФЦМгШыЃЌMЁЂNЁЂPЁЂQЕФМюаддіЧПЃЌдђc(H+)МѕаЁЃЌЫљвдШмвКжа
ЃЌОнЭМПЩжЊЫцзХЧтбѕЛЏФЦМгШыЃЌMЁЂNЁЂPЁЂQЕФМюаддіЧПЃЌдђc(H+)МѕаЁЃЌЫљвдШмвКжа![]() ЃЌЙЪCе§ШЗЃЛ
ЃЌЙЪCе§ШЗЃЛ
DЃЎЫЎЕчРыГіЕФOH-ХЈЖШдНДѓЫЎЕФЕчРыГЬЖШдНДѓЃЌМДЭМжазнзјБъдНаЁЫЎЕФЕчРыГЬЖШдНДѓЃЌЫљвдЫЎЕФЕчРыГЬЖШ:P>N=Q>MЃЛЮТЖШЮДжЊЃЌЮоЗЈШЗЖЈЫЎЕФЕчРыЦНКтГЃЪ§ЃЌЫљвдЮоЗЈШЗЖЈaЕФжЕЃЌЙЪDДэЮѓЃЛ
ЙЪД№АИЮЊDЁЃ

ЁОЬтФПЁПЕЊКЭЕЊЕФЛЏКЯЮядкЙЄХЉвЕЩњВњЁЂЙњЗРКЭЩњЛюжаЖМгаМЋЦфЙуЗКЕФгУЭОЁЃЧыЛиД№ЯТСагыЕЊдЊЫигаЙиЕФЮЪЬтЃК
ЃЈ1ЃЉбЧЯѕЫсТШ(НсЙЙЪНЮЊCl-N=O)ЪЧгаЛњКЯГЩжаЕФживЊЪдМСЃЌПЩгЩCl2КЭNOдквЛЖЈЬѕМўЯТЭЈЙ§вдЯТЗДгІжЦЕУЃК2NO+Cl2=2ClNOЃЌвбжЊМИжжЛЏбЇМќЕФМќФмЪ§ОнШчЯТБэЫљЪОЃК
ЛЏбЇМќ | Cl-Cl | Cl-N | N=O | N=O(NO) |
МќФм(kJЁЄmol-1) | 243 | a | 607 | 630 |
ЧыИљОнБэжаЪ§ОнМЦЫуЃК2NO(g)+Cl2(g)=2ClNO(g)ЁїH=___kJmol-1ЁЃ
ЃЈ2ЃЉЮТЖШвЛЖЈЪБЃЌдквЛИіЬхЛ§ЮЊ1LЕФУмБеШнЦїжаЭЈШы2molNOКЭ1molCl2ЃЌ10minЪБClNOЬхЛ§еМЦјЬхзмЬхЛ§40%ЃЈЦјЬхЕФЁАЬхЛ§ЗжЪ§ЁБМДЁАЮяжЪЕФСПЗжЪ§ЁБЃЉЃЌдђЗДгІПЊЪМЕН10minФкNOЕФЦНОљЗДгІЫйТЪЃКv(NO)=___molЁЄL-1ЁЄmin-1
ЃЈ3ЃЉвдNH3ЮЊЛЙдМСдкЭбЯѕзАжУжаЯћГ§бЬЦјжаЕФЕЊбѕЛЏЮяЃЌ
жїЗДгІЮЊ4NH3(g)+4NO(g)+O2(g)=4N2(g)+6H2O(g) ЁїH1
ИБЗДгІЃК4NH3(g)+3O2(g)=2N2(g)+6H2O(g) ЁїH2=Ѓ1267.1kJ/mol
4NH3(g)+5O2(g)=4NO(g)+6H2O(g) ЁїH3=Ѓ907.3kJ/mol
ЂйЁїH1=___ЁЃ
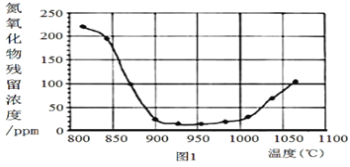
ЂкНЋбЬЦјАДвЛЖЈЕФСїЫйЭЈЙ§ЭбЯѕзАжУЃЌВтЕУГіПкNOЕФХЈЖШгыЮТЖШЕФЙиЯЕШчЭМ1ЃЌЪдЗжЮіЭбЯѕЕФЪЪвЫЮТЖШЪЧ___ЃЈЬюађКХЃЉ
a.<850Ёц b.900~1000Ёц c.>1050Ёц
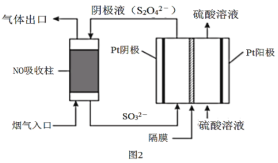
ЃЈ4ЃЉвдСЌЖўбЧСђЫсбЮ(S2O42-)ЮЊЛЙдМСЭбГ§бЬЦјжаЕФNOЃЌВЂЭЈЙ§ЕчНтдйЩњЃЌзАжУШчЭМ2ЁЃвѕМЋЕФЕчМЋЗДгІЪНЮЊ___ЁЃ
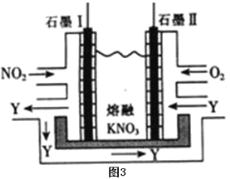
ЃЈ5ЃЉРћгУЕчЛЏбЇдРэЃЌНЋNO2ЁЂO2КЭШлШкKNO3жЦГЩШМСЯЕчГиЃЌзАжУШчЭМ3ЫљЪОЃЌЕчГиЙЄзїЪБЃЌNO2зЊБфГЩТЬЩЋЯѕЛЏМСY(N2O5)ЃЌПЩбЛЗЪЙгУЃЌдђЪЏФЋЂёИННќЗЂЩњЕФЕчМЋЗДгІЪНЮЊЃК___ЁЃ
ЁОЬтФПЁПИљОндгЛЏЙьЕРРэТлКЭМлЕчзгЖдЛЅГтРэТлФЃаЭХаЖЯЃЌЯТСаЗжзгЛђРызгЕФжааФдзгЕФдгЛЏЗНЪНМАПеМфЙЙаЭе§ШЗЕФЪЧЃЈ ЃЉ
бЁЯю | ЗжзгЛђРызг | жааФдзгдгЛЏЗНЪН | МлЕчзгЖдЛЅГтРэТлФЃаЭ | ЗжзгЛђРызгЕФПеМфЙЙаЭ |
A |
|
| жБЯпаЮ | жБЯпаЮ |
B |
|
| ЦНУцШ§НЧаЮ | Ш§НЧзЖаЮ |
C |
|
| ЫФУцЬхаЮ | ЦНУцШ§НЧаЮ |
D |
|
| ЫФУцЬхаЮ | е§ЫФУцЬхаЮ |
A.AB.BC.CD.D