��Ŀ����
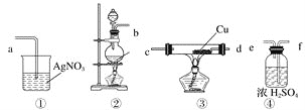
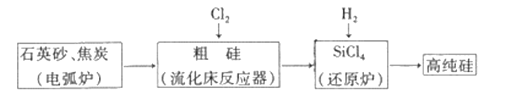
����Ŀ����������(CaO2)����ˮ�������ᣬ���������Լ���ҽ�÷���������������ʵ���ҳ���CaCO3Ϊԭ���Ʊ��������ƣ�������ͼ��
![]()
����˵������ȷ����
A. ��μ���ϡ�������Һ��е������dz�ȥ��Һ�ж����CO2
B. ���백ˮ��˫��ˮ��ķ�ӦΪ��CaCl2��2NH3��H2O��H2O2��CaO2����2NH4Cl��2H2O
C. ����CaO2�ķ�Ӧ��Ҫ�ڱ�ԡ�½��е�ԭ�����¶ȹ���ʱ��������ֽ�
D. ��Ʒ����������ˮ����ˮ�Ҵ�ϴ�ӣ������Ҵ�ϴ�ӵ�Ŀ����Ϊ�˳�ȥ��������NH4Cl����
���𰸡�D
��������
�����̿�֪��̼��������������Һ��У����Գ�ȥ��Һ���ܽ�Ķ�����̼���壬��ֹ������̼�백����Ӧ�����˺���Һ���Ȼ��ơ���ˮ���������ⷴӦ����CaO2��NH4Cl��ˮ����Ӧ�ڱ�ԡ�½��У��ɷ�ֹ��������ֽ⣬�ٹ��ˣ�ϴ�ӵõ��������ư�ɫ���壬�ݴ˷������
A����μ���ϡ�������Һ��е������dz�ȥ��Һ������̼����ֹ�ٺ���ʵ���ж�����̼�백����Ӧ����A��ȷ��
B����Һ�м��백ˮ��˫��ˮ����CaO2����Ӧ�ķ���ʽΪ��CaCl2+2NH3H2O+H2O2�TCaO2��+2NH4Cl+2H2O����B��ȷ��
C���¶ȹ���ʱ��������ֽ⣬Ӱ�췴Ӧ���ʣ�������CaO2�ķ�Ӧ��Ҫ�ڱ�ԡ�½��У���C��ȷ��
D�����˵õ��İ�ɫ�ᾧΪCaO2������ˮ��������ˮϴ�Ӻ�Ӧ�����Ҵ�ϴ����ȥ���ᾧ����ˮ�֣�ͬʱ�ɷ�ֹ�����ܽ⣬��D����
��ѡD��