��Ŀ����
����Ŀ����I��LiH�����ɴ���ȼ�ϣ���֪���з�Ӧ��
��2Li(s)+H2(g)===2LiH(s) ��H��-182 kJ��mol-1��
��2H2(g)+O2(g)===2H2O(l) ��H��-572 kJ��mol-1��
��4Li(s)+O2(g)===2Li2O(s) ��H��-1196 kJ��mol-1��
��д��LiH��O2��ȼ�յ��Ȼ�ѧ����ʽ��__________________________________________��
��II������H2��CO2�����״���ij�¶��£����ݻ�Ϊ2L���ܱ������г���1molCO2��3.25mol H2����һ�������·�Ӧ�����CO2��CH3OH��g����H2O��g�������ʵ�����n����ʱ��ı仯��ϵ��ͼ��ʾ��
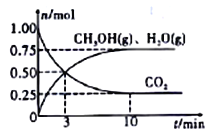
��1���ӷ�Ӧ��ʼ��3minʱ��������ƽ����Ӧ����v(H2)=____________��
��2�����д�ʩ��һ����ʹCO2��ת�����������_______________������ţ���
A.��ԭ�������ٳ���1mol CO2 B.��ԭ�������ٳ���1mol H2
C.��ԭ�������ٳ���1mol He D.ʹ�ø���Ч�Ĵ���
E. ��С�������ݻ� F. ��ˮ��������ϵ�з����
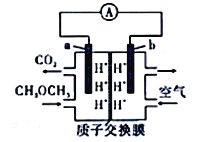
��III��ͼ�Ǽ״�ȼ�ϵ�ع�����ʾ��ͼ������A��B��D��Ϊʯī�缫��CΪͭ�缫������һ��ʱ��Ͽ�K����ʱA��B�����ϲ��������������ͬ��
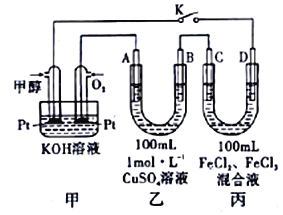
��1�����и����ĵ缫��ӦʽΪ_______________________________��
��2������A�������������ڱ�״���µ����Ϊ_______________________��
��IV����֪������CN-��ˮ�ⳣ��Kb=1.61��10-5��
��1�������£��������ʵ���Ũ�ȵ�HCN��NaCN�Ļ����Һ��_________����ᡱ������С����ԣ�c(CN-)_______���>����<����=����c(HCN)��
��2�������£�����c molL-1������0.61 molL-1KCN��Һ�������Ϻ�ǡ�õõ�������Һ����c=________��С�������4λ��������
���𰸡� 2LiH(s)+O2(g)==Li2O(s)+H2(l) ��H=-702kJmol-1 0.25mol��L-1��min-l BEF CH3OH-6e-+8OH-==CO32-+6H2O 2.24L �� �� 0.6162
��������������Ҫ���黯ѧ��Ӧԭ���ۺϿ��顣
��I����/2+��/2-�ٵ�LiH��O2��ȼ�յ��Ȼ�ѧ����ʽ��2LiH(s)+O2(g)==Li2O(s)+H2(l) ��H=-702kJmol-1��
��II��������Ӧ��3H2��g��+CO2��g��![]() CH3OH��g��+H2O��g����
CH3OH��g��+H2O��g����
��1���ӷ�Ӧ��ʼ��3minʱ��������ƽ����Ӧ����v(H2)= 3v(CO2)=3��0.50/2/3 mol��L-1��min-l =0.25 mol��L-1��min-l��
��2��A.��ԭ�������ٳ���1molCO2 ��CO2��ת���ʼ�С��B.��ԭ�������ٳ���1molH2����Ӧ��Ũ������ƽ�����ƣ���ʹCO2��ת����������C.��ԭ�������ٳ���1molHe��ƽ�ⲻ�ƶ���CO2��ת���ʲ��䣻D.ʹ�ø���Ч�Ĵ�����ƽ�ⲻ�ƶ���CO2��ת���ʲ��䣻E. ��С�������ݻ�����ѹƽ�����ƣ���ʹCO2��ת����������F. ��ˮ��������ϵ�з������������Ũ�ȼ�С��ƽ�����ƣ���ʹCO2��ת��������ѡBEF��
��III����1������ͨ��״��ĵ缫Ϊ�����������ĵ缫��ӦʽΪCH3OH-6e-+8OH-==CO32-+6H2O��
��2��B��������0.1molCu��������������A��������������A��B�����ϲ��������������ͬʱ��A������0.1mol����������A�������������ڱ�״���µ����Ϊ2.24L��
��IV����1�������£��������ʵ���Ũ�ȵ�HCN��NaCN�Ļ����Һ�Լ��ԣ�˵��NaCN��ˮ��̶ȴ���HCN�ĵ���̶ȣ�����c(CN-)<c(HCN)��
��2�������Һ�����ԣ�˵��c(CN-)<c(HCN)��c(HCN) = c molL-1��c(CN-)=(0.61-c) c molL-1��c(OH-)=1��10-7molL-1��Kb=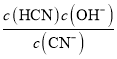 =1.61��10-5����c=0.6162��
=1.61��10-5����c=0.6162��

����Ŀ������ʵ������Ͷ�Ӧ�����ӷ���ʽ����ȷ������ ��
ѡ�� | ʵ����� | ���ӷ���ʽ |
A | ���廯������Һ�еμ���ˮ,֤����ԭ�ԣ�Fe2+>Br- | Cl2+2Br-=Br2+2Cl- |
B | �ó���ʯ��ˮ����K2SO3��Һ��KHSO3��Һ | Ca2++SO32-=CaSO3�� |
C | �ñ���Na2CO3��Һ�����Թ��е������ | CaSO4+CO32- |
D | ��Al2(SO4)3��Һ�еμӰ�ˮ�Ʊ��������� | Al3++3OH-=Al(OH)3�� |
A. A B. B C. C D. D
����Ŀ�����ǵ����Ϻ����ḻ��Ԫ�أ������仯������о�������������������Ҫ���塣
��1����ͼ��1 mol NO2��1 mol CO��Ӧ����CO2��NO�����������仯ʾ��ͼ��д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ��
��2����֪��
N2��g��+O2��g��=2 NO��g�� ��H=+180 kJ��mol-1
2NO��g��+2 CO��g��=N2��g��+2 CO2��g����H=��746 kJ��mol-1
��ӦCO��g��+1/2O2��g��=CO2��g���ġ�H=kJ��mol-1��
��3����һ�̶��ݻ�Ϊ2L���ܱ������ڼ���0.2 mol��N2��0.6 mol��H2 �� ��һ�������·������·�Ӧ��N2(g)+3H2(g) ![]() 2NH3(g)��H<0������5minʱ�ﵽƽ�⣬��ʱ���NH3�����ʵ���Ϊ0.2mol����ǰ5min��ƽ����Ӧ����v��N2��Ϊ �� ƽ��ʱH2��ת����Ϊ �� �÷�Ӧ��ƽ�ⳣ��K=(����������һλС��)��
2NH3(g)��H<0������5minʱ�ﵽƽ�⣬��ʱ���NH3�����ʵ���Ϊ0.2mol����ǰ5min��ƽ����Ӧ����v��N2��Ϊ �� ƽ��ʱH2��ת����Ϊ �� �÷�Ӧ��ƽ�ⳣ��K=(����������һλС��)��
��4���ڹ̶�������ܱ������У�1.0��103kPaʱ����ӦN2(g)+3H2(g) ![]() 2NH3(g) ��H <0��ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±�����K1K2����д��>������=����<����
2NH3(g) ��H <0��ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±�����K1K2����д��>������=����<����
T/K | 298 | 398 | 498 |
ƽ�ⳣ��K | 51 | K1 | K2 |
��5�������һ�����ܱ���������˵���ϳɰ���Ӧһ���ﵽƽ��״̬����������ĸ��
a��������N2��H2��NH3��Ũ��֮��Ϊ1�U3�U2
b��NH3��Ũ�ȱ��ֲ���
c��������ѹǿ���ֲ���
d�����������ܶȱ��ֲ���