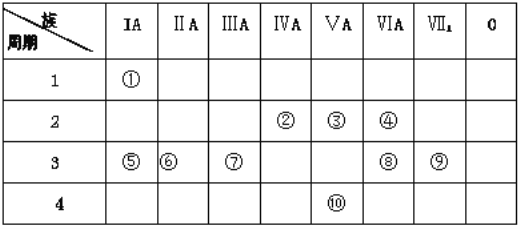
��Ŀ����
����Ŀ��SO2�ǹ���������ʹ�õ�һ��ʳƷ���Ӽ�����ʳƷ��ҵ�з����Ż�ɫ��������Ư�Ϳ����������á��ҹ�����SO2������������ȷ�涨(��ͼ��ʾ)��

ij�о�С����ͼװ�ö����ۻƻ����е�SO2���вⶨ�����У���ҺXΪ�ƻ��˽��г�ֽ��ݺ�����dz��ɫ����Һ��YΪ������Լ���(Y��Ũ�ȼ������δ���)�������Ϸ��֣���ˮ��Һ�����ļ���Ԫ����Ҫ��SO32-��ʽ���ڣ���SO2��SO32-��ѧ�������ơ���ˣ���С��ͬѧ��SO2�IJⶨ����ת��Ϊ��Һ��SO32-�IJⶨ��
(1)��ϻ�ѧ֪ʶ���ͽ���ҺX�д���SO32-��ԭ����______��
(2)��ͬѧԤ�����Na2S��Һ��֤X���Ƿ���SO32-����Ԥ��Ӧ�õ���Na2S��______�ԡ��������Ϸ��ָ÷�Ӧ��Ϊ���ӣ����δ����һ����֤��
(3)��ͬѧΪ��֤X���Ƿ���SO32-��������ʵ��i��
ʵ����� | �Լ�Y | ���� |
i | ����KMnO4��Һ | �� |
����ʵ��i ������ó����ۣ�X�к���SO32-��ʵ��i��������________��SO32-�ڷ�Ӧ��ת��Ϊ_________��
(4)��ͬѧ��ΪҲ������H2O2��Һ����֤X���Ƿ���SO32-��������ʵ��ii��
ʵ����� | ���� | ���� |
ii | a����X�м�������H2O2��Һ���� | ���������� |
b�������μ� ���� | ���������� | |
c���ٵμ� ���� | ��ɫ���� |
ͨ��ʵ��ii��֤��X�к���SO32-��д������b��c�еμ��Լ��Ļ�ѧʽ____�� _____ ��
(5)��ͬѧ��Ϊ����ʵ�鷽�����Ͻ�������˶Ա�ʵ������֤����X�к���SO32-��ʵ�����ķ�����(�Ӳ����������۷�������)________ ��
(6)Ϊ�ⶨ���ۻƻ�����SO2����������ʵ������
ʵ����� | ���� |
�� | a����50�˻ƻ������飬��һ��Ũ������������Һ������ b����ϡ�����ữ�� c���������ָʾ���� d����Ũ��Ϊ0.01mol/L��I2��Һ�ζ�������0.01mol/L��I2��Һ5.00 mL�� |
�������Ϸ��֣���Ԫ�صij�����̬��-1��0��+1��+3��+5��+7��
�� ���ƻ���������������Һ���ݵ�Ŀ����(�û�ѧ����ʽ��ʾ)__________��
�� д���ζ���Ӧ�Ļ�ѧ����ʽ____________��
�� �����ۻƻ�����SO2��������________����/ǧ�ˡ�
���𰸡�SO2��H2O��Ӧ����H2SO3, H2SO3�ɵ������H+��SO32- ��ԭ ��ɫ SO42- ���� BaCl2��Һ ȡ��ҺX���μ����ᣬ�ٵμ��Ȼ�����Һ����������ɫ���������ʵ������֤��X����SO32- SO2+2NaOH = Na2SO3+H2O Na2SO3+I2+H2O=Na2SO4+2HI 64
��������
(1)����ҺX�д���SO32-��ԭ����SO2��H2O��Ӧ����H2SO3, H2SO3�ɵ������H+��SO32-��
(2)��ΪNa2S��SO32-���Է�����Ӧ��2S2-+ SO32-+6H+=3S![]() +3H2O���÷�Ӧ��Na2S����ԭ�����𰸣���ԭ����
+3H2O���÷�Ӧ��Na2S����ԭ�����𰸣���ԭ����
��3��X�к��е�SO32-���������ԣ����л�ԭ�ԣ�����������KMnO4��Һ��Ӧ����Һ����ɫ�������ķ�ӦΪ5SO32-+ 2MnO4-+6H+=5 SO42-+2 Mn2++3H2O��SO32-�ڷ�Ӧ��ת��ΪSO42-;�𰸣���ɫ��SO42-��
(4) X�к���SO32-����������H2O2��Һ������ӦΪ��SO32- +H2O2= SO42-+H2O,������Һ�м���ϡ���ᣬû�з�Ӧ�������ټ�BaCl2��Һ���ɰ�ɫ�������������Բ���b��c�еμӵ��Լ�Ϊ�����BaCl2��Һ���𰸣����� �� BaCl2��Һ��
(5)��ͬѧ��Ϊ����ʵ�鷽�����Ͻ�������˶Ա�ʵ������֤����X�к���SO32-��ʵ�����ķ�������ȡ��ҺX���μ����ᣬ�ٵμ��Ȼ�����Һ����������ɫ���������ʵ������֤��X����SO32- ��
(6)�� ���ƻ���������������Һ������Ϊ�˳�ȥ�ƻ�����SO2 ����Ӧ�Ļ�ѧ����ʽ��SO2+2NaOH = Na2SO3+H2O���𰸣�SO2+2NaOH = Na2SO3+H2O��
����Ũ��Ϊ0.01mol/L��I2��Һ�ζ����ۻƻ����е���ȡҺ���ζ���Ӧ�Ļ�ѧ����ʽ��Na2SO3+I2+H2O=Na2SO4+2HI���𰸣�Na2SO3+I2+H2O=Na2SO4+2HI��
����Ũ��Ϊ0.01mol/L��I2��Һ�ζ�������0.01mol/L��I2��Һ5.00 mL����Ϸ�Ӧ����ʽ��Na2SO3+I2+H2O=Na2SO4+2HI��n(SO2)=n (Na2SO3)=n(I2)= 0.01mol/L![]() /1000mL
/1000mL![]() L-1=5
L-1=5![]() 10-5mol,���ۻƻ�����SO2����������5
10-5mol,���ۻƻ�����SO2����������5![]() 10-5mol
10-5mol![]() 64g/mol=3.2
64g/mol=3.2![]() 10-3g���������ۻƻ�����SO2����=��3.2
10-3g���������ۻƻ�����SO2����=��3.2![]() 10-3g
10-3g![]() 1000��
1000��![]() 50/1000=64����/ǧ��;�𰸣�64��
50/1000=64����/ǧ��;�𰸣�64��

 ��У����ϵ�д�
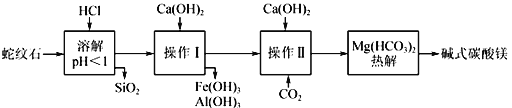
��У����ϵ�д�����Ŀ��Li4Ti5O12��LiFePO4��������ӵ�صĵ缫���ϣ���������������Ҫ�ɷ�ΪFeTiO3������������MgO��SiO2�����ʣ����Ʊ��������������£�

�ش��������⣺
��1������1Ϊ_______________������AΪ_________________������Һ���м���˫��ˮ��������___________________________________��
��2���������������Ҫ��TiOCl42����ʽ���ڣ�д����Ӧ��Ӧ�����ӷ���ʽ__________��
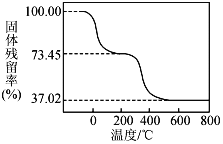
��3��TiO2��xH2O������˫��ˮ����ˮ��Ӧ40 min����ʵ�������±���ʾ��
�¶�/�� | 30 | 35 | 40 | 45 | 50 |
TiO2��xH2Oת����% | 92 | 95 | 97 | 93 | 88 |
����40��ʱTiO2��xH2Oת������ߵ�ԭ��_______________________________��
��4��������Һ�ڡ���c(Mg2+)=0.02 mol/L������˫��ˮ�����ᣨ����Һ�������1������ʹFe3+ǡ�ó�����ȫ����Һ��c(Fe3+)=1��10-5 mol/L����ʱ�Ƿ���Mg3(PO4)2�������ɣ�_____________________________________________����ʽ���㣩��FePO4��Mg3(PO4)2��Ksp�ֱ�Ϊ1.3��10-22��1.0��10-24��