��Ŀ����
7��NO2����ˮ����HNO3��NO����ҵ��������һ��Ӧԭ����ȡHNO3��3NO2+H2O=2HNO3+NO ����һ�����գ�
����һ��NO2��������ֻ��$\frac{2}{3}$��������$\frac{1}{3}$��NO��Ϊ�˸��������NO2����ҵ��ͨ�������ɵ�NO�ٴ�����ΪNO2���ٴ���ˮ����
2NO+O2=2NO2 ����һ��ͨO2��
3NO2+H2O=2HNO3+NO ���ڶ������գ�
���ѭ��������ʹNO2ȫ��ת��ΪHNO3��
����3��NO2�������������������ѭ���������գ�
��1����ڶ������պ�NO2�������հٷ���Ϊ$\frac{8}{9}$�������÷�����ʾ����ͬ�����ڶ���ͨ��O2�����Ϊ$\frac{1}{6}$�������������պ�ʣ��NO$\frac{1}{9}$����
��2����n�����պ�ʣ��NO�����Ϊ$\frac{1}{3{\;}^{n-1}}$�����ú�n�Ĵ���ʽ��ʾ����
��3�����Ҫʹ3��NO2ǡ����ȫת��ΪHNO3������ͨ��O2�������Ϊ$\frac{3}{4}$�����������������������ͬ�����²ⶨ��
���� ��1����������3��NO2�����3NO2+H2O=2HNO3+NO ����һ�����գ�2NO+O2=2NO2 ����һ��ͨO2��3NO2+H2O=2HNO3+NO ���ڶ������գ����м��㣻
��2������3NO2+H2O=2HNO3+NO��ÿ����һ��ʣ��NO��Ϊԭ����$\frac{1}{3}$���㣻
��3����3NO2+H2O=2HNO3+NO ����һ�����գ�2NO+O2=2NO2 ����һ��ͨO2����������ʽ�������ϵõ�4NO2+O2+2H2O=4HNO3 ���м��㣻
��� �⣺��1������3��NO2������3NO2+H2O=2HNO3+NO ����һ�����գ�����NO1L������2NO+O2=2NO2��������1��NO2������3NO2+H2O=2HNO3+NO ���ڶ������գ������ʣ��$\frac{1}{3}$LNO�����Եڶ������պ�NO2�������հٷ���Ϊ$\frac{3-\frac{1}{3}}{3}$=$\frac{8}{9}$��$\frac{1}{3}$LNO�ڶ���ͨ��O2��������2NO+O2=2NO2�����Եڶ���ͨ��O2�����Ϊ$\frac{1}{3}$L��$\frac{1}{2}$=$\frac{1}{6}$L������3NO2+H2O=2HNO3+NO��ÿ����һ��ʣ��NO��Ϊԭ����$\frac{1}{3}$�����Ե��������պ�ʣ��NOΪ$\frac{1}{3}$��$\frac{1}{3}$=$\frac{1}{9}$���ʴ�Ϊ��$\frac{8}{9}$��$\frac{1}{6}$��$\frac{1}{9}$��
��2����Ϊ3NO2+H2O=2HNO3+NO��ÿ����һ��ʣ��NO��Ϊԭ����$\frac{1}{3}$�����Ե�n�����պ�ʣ��NO�����Ϊ$\frac{1}{3{\;}^{n-1}}$���ʴ�Ϊ��$\frac{1}{3{\;}^{n-1}}$��
��3����3NO2+H2O=2HNO3+NO ����һ�����գ�2NO+O2=2NO2 ����һ��ͨO2����������ʽ�������ϵõ�4NO2+O2+2H2O=4HNO3������Ҫʹ3��NO2ǡ����ȫת��ΪHNO3������ͨ��O2�������Ϊ$\frac{1}{4}$��3L=$\frac{3}{4}$L���ʴ�Ϊ��$\frac{3}{4}$��
���� ���⿼�黯ѧ��Ӧ����ʽ�ļ��㣬Ϊ��Ƶ���㣬���շ����Ļ�ѧ��ӦΪ���Ĺؼ���ע�ⷽ��ʽ��Ӧ���ɺ������ǽ���ؼ�����Ŀ�Ѷ��еȣ�

ʪ���Ʊ�����Ҫ��Ӧ����Ϊ��2Fe��OH��3+3NaClO+4NaOH=2Na2FeO4+3NaCl+5H2O
�ɷ��Ʊ�����Ҫ��Ӧ����Ϊ��2FeSO4+4Na2O2=2Na2FeO4+2Na2SO4
�����йظ÷�Ӧ��˵��������ǣ�������
| A�� | ����Ӧ��Na2FeO4��Ϊ�������� | |
| B�� | Na2FeO4��ǿ�����ԣ�������ɱ�����仹ԭ����������ˮ������ | |
| C�� | �ɷ���ÿ����1mol Na2FeO4ת��3mol���� | |
| D�� | ���������£�NaClO�����Դ���Na2FeO4 |
| A�� | ����ˮ���γɵ�Al��OH��3����������ˮ�������������ˮ�ľ��� | |
| B�� | �ں������������п�飬�ɼ�������ĸ�ʴ���� | |
| C�� | MgO���۵�ܸߣ��������������²��� | |
| D�� | ���AlCl3������Һ�����Ƶý����� |
| NaOH��ʼ���� | NaOH�յ���� | |
| ��һ�� | 0.40mL | 18.50mL |
| �ڶ��� | 1.30mL | 18.05mL |
| ������ | 3.10mL | 21.20mL |
��2�������������ݿ��Լ������������ʵ���Ũ��Ϊ0.1448mol/L��
��3�����²�����ɲⶨ���ƫ�ߵ�ԭ�������CD��
A���ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�������������ȷ
B��ʢװδ֪Һ����ƿ������ˮϴ����δ�ô���Һ��ϴ
C���ζ����յ����ʱ���ֵζ��ܼ��촦����һ����Һ
D��δ�ñ�Һ��ϴ��ʽ�ζ��ܣ�
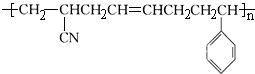 ��������˸߷��Ӳ�����ص�˵����ȷ���ǣ�������
��������˸߷��Ӳ�����ص�˵����ȷ���ǣ�������| A�� | �ϳɸø߷��Ӳ��ϵķ�Ӧ�����۷�Ӧ | |
| B�� | �ø߷��Ӳ����������ֵ���ۺ϶��ɵ� | |
| C�� | �ϳɸø߷��Ӳ��ϵIJ��ֵ��岻��ʹ��ˮ�����Ը��������Һ��ɫ | |
| D�� | �ø߷��Ӳ��������ͽṹ�߷��ӣ������ȹ��� |
| A�� | Na+��K+��SO42-��HCO3- | B�� | Cu2+��K+��SO42-��NO3- | ||
| C�� | Fe3+��K+��SO42-��Cl- | D�� | Na+��K+��Cl-��NO3- |
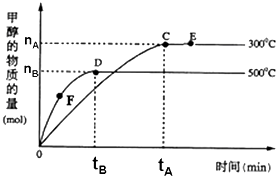 һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״���CO��g��+2H2��g��?CH3OH��g�����������⣬����˵����ȷ���ǣ�������
һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״���CO��g��+2H2��g��?CH3OH��g�����������⣬����˵����ȷ���ǣ�������| A�� | ����Ӧ�ġ�H��0 | |
| B�� | ����������������ʵ���E�����D�� | |
| C�� | F�������Ӧ���ʴ����淴Ӧ���� | |
| D�� | v���״���=$\frac{{n}_{B}}{3{t}_{B}}$mol/��L•min����ʾ500��ʱ��Ӧ��D������� |
| A�� | ʳ��ˮ | B�� | �ռ���Һ | C�� | ������Һ | D�� | ϡ���� |
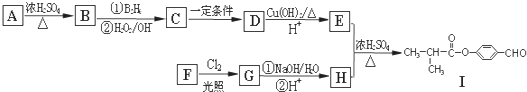
 ��
�� ��E��H��Ӧ����I�ķ���ʽΪ
��E��H��Ӧ����I�ķ���ʽΪ ��
��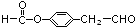 ��
��