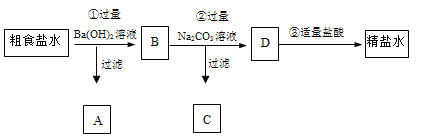
��Ŀ����
����Ŀ��ijͬѧ̽��Ba(OH)2��H2SO4��Ӧ��ʵ�ʣ�������ͼװ�ý���ʵ�顣��20 ml 0.01 mol/L Ba(OH)2 ��Һ�е��뼸�η�̪��Һ��Ȼ��������������μ���2 ml 0.2 mol/L H2SO4��Һ��

��1��ʵ���������Һ�е�����Ϊ________�� ________��
��2���÷�Ӧ�����ӷ���ʽ��__________________��
��3��������Ӧ��������Һ�絼�ʱ仯ʾ��ͼ__________________��
��4�����ͷ�Ӧ��������Һ�絼�ʳ��������仯��ԭ��___��
���𰸡�������ɫ���� �ձ�����Һ�ɺ�ɫ��Ϊ��ɫ Ba2++2OH��+2H++SO42��= BaSO4��+2H2O 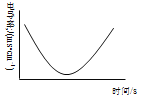 ���ż������ᣬBa2+��SO42����Ӧ����BaSO4������OH����H+��Ӧ����H2O����Һ������Ũ�ȼ�С����˵絼���½���Ba(OH)2��Ӧ�������μ����ᣬ��Һ��H+ ��SO42����Ũ�����ӣ���Һ�絼������
���ż������ᣬBa2+��SO42����Ӧ����BaSO4������OH����H+��Ӧ����H2O����Һ������Ũ�ȼ�С����˵絼���½���Ba(OH)2��Ӧ�������μ����ᣬ��Һ��H+ ��SO42����Ũ�����ӣ���Һ�絼������
��������
��1����̪�����죬��������������������Ӧ������Һ������������Ũ�ȵı仯���
��2������������������Ӧ�������ᱵ��ˮ�����ӷ�ӦΪ��Ba2++2OH��+2H++SO42��= BaSO4��+2H2O��
��3����Һ�ĵ���������Һ������Ũ�ȴ�С�йأ�����Ũ��Խ����Խǿ����֮Խ��������������������Һ�μ��������Һ������Ũ�ȵı仯����������Һ�ĵ����ԣ�
��4������������������Һ�μ��������Һ������Ũ�ȵı仯����������Һ�ĵ����ԡ�
��1��ԭ�ձ�����ǿ��������������̪�����죬����������������Ӧ���ɰ�ɫ������ͬʱ��Һ������������Ũ�ȼ�С������������Һ�ĵμӣ�����Һ�е����������������꣬��Һ��Ϊ��ɫ���ʴ�Ϊ��������ɫ�������ձ�����Һ�ɺ�ɫ��Ϊ��ɫ��
��2������������������Ӧ�������ᱵ��ˮ�����ӷ�ӦΪ��Ba2++2OH��+2H++SO42��= BaSO4��+2H2O���ʴ�Ϊ��Ba2++2OH��+2H++SO42��= BaSO4��+2H2O��
��3����Һ�ĵ���������Һ������Ũ�ȴ�С�йأ�����Ũ��Խ����Խǿ����֮Խ����Ϊ�μ�����ǰ����Һ��ֻ��ǿ����������������������������������ĵμӣ�����������������Ӧ�������ᱵ��ˮ����Һ������Ũ�ȼ�С����Һ�����Խ��ͣ������ǡ����ȫ�к�ʱ����Һ������Ũ����С����������С���ټ����μ����ᣬ��Һ������Ũ�����������Һ������������ǿ����Ӧ��������Һ�絼�ʱ仯ʾ��ͼΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��4�����ż������ᣬBa2+��SO42����Ӧ����BaSO4������OH����H+��Ӧ����H2O����Һ������Ũ�ȼ�С����˵絼���½���Ba(OH)2��Ӧ�������μ����ᣬ��Һ��H+ ��SO42����Ũ�����ӣ���Һ�絼�����ӣ��ʴ�Ϊ�����ż������ᣬBa2+��SO42����Ӧ����BaSO4������OH����H+��Ӧ����H2O����Һ������Ũ�ȼ�С����˵絼���½���Ba(OH)2��Ӧ�������μ����ᣬ��Һ��H+ ��SO42����Ũ�����ӣ���Һ�絼�����ӡ�