��Ŀ����
Fe2O3��Cu2O���Ǻ�ɫ��ĩ�����������ϡ�ijУ�о���ѧϰС��ͨ��ʵ��̽��һ����ɫ��ĩ��Fe2O3��Cu2O�������̽���������£�
�������ϣ�Cu2O����ϡ���������Cu��CuSO4
������裺����1����ɫ��ĩ��Fe2O3
����2����ɫ��ĩ��Cu2O
����3����ɫ��ĩ��Fe2O3��Cu2O�Ļ����
���̽��ʵ�飺ȡ������ĩ��������ϡ�����У���������Һ���ٵμ� KSCN �Լ���
��1��������1��������ʵ�������� ��
��2�����μ� KSCN �Լ�����Һ������������֤��ԭ�����ĩ��һ������Fe2O3������Ϊ����˵�������� ���������������������
��3���������ĩ��ȫ�ܽ⼴��Һ���������, �μ�KSCN �Լ�ʱ��Һ����������, ��֤��ԭ�����ĩ�� ��д����ط�Ӧ�����ӷ���ʽ��
��
�������ϣ�Cu2O����ϡ���������Cu��CuSO4
������裺����1����ɫ��ĩ��Fe2O3
����2����ɫ��ĩ��Cu2O
����3����ɫ��ĩ��Fe2O3��Cu2O�Ļ����
���̽��ʵ�飺ȡ������ĩ��������ϡ�����У���������Һ���ٵμ� KSCN �Լ���
��1��������1��������ʵ�������� ��
��2�����μ� KSCN �Լ�����Һ������������֤��ԭ�����ĩ��һ������Fe2O3������Ϊ����˵�������� ���������������������
��3���������ĩ��ȫ�ܽ⼴��Һ���������, �μ�KSCN �Լ�ʱ��Һ����������, ��֤��ԭ�����ĩ�� ��д����ط�Ӧ�����ӷ���ʽ��
��
��1����Һ��ΪѪ��ɫ
��2����������Cu�ܽ�Fe3+��ԭΪFe2+
��3��Fe2O3��Cu2O�Ļ����
Fe2O3+6H+ = 2Fe3++3H2O �� Cu2O+2H+ =" Cu" + Cu2+ + H2O�� 2 Fe3+ + Cu =" 2" Fe2+ + Cu2+
��2����������Cu�ܽ�Fe3+��ԭΪFe2+
��3��Fe2O3��Cu2O�Ļ����
Fe2O3+6H+ = 2Fe3++3H2O �� Cu2O+2H+ =" Cu" + Cu2+ + H2O�� 2 Fe3+ + Cu =" 2" Fe2+ + Cu2+
��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
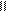 ������һ����̽��С�������ѧ֪ʶ���������Ҵ���ˮ�����ӡ�̼�ᣨ HO��C��OH��������������ǻ�����ԭ�ӵĻ����ԡ� O
������һ����̽��С�������ѧ֪ʶ���������Ҵ���ˮ�����ӡ�̼�ᣨ HO��C��OH��������������ǻ�����ԭ�ӵĻ����ԡ� O 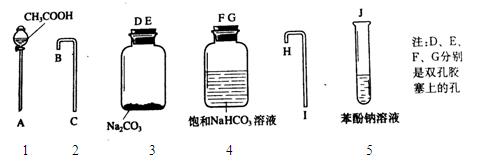
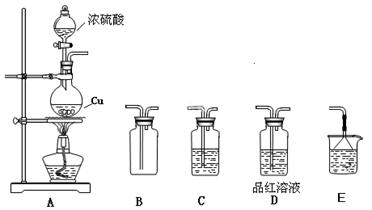
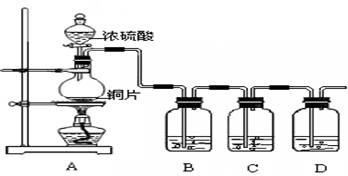
 2Fe+ Al2O3��ij�о�С����ʵ�������ü���װ�ý������ȷ�Ӧ���������ɵ��������ɵĺ�ɫӲ�顣С���Ա�Ʋ���Ҫԭ���Dz����������ʽ϶࣬����һ��̽���ʺ�ɫӲ�����ɡ�
2Fe+ Al2O3��ij�о�С����ʵ�������ü���װ�ý������ȷ�Ӧ���������ɵ��������ɵĺ�ɫӲ�顣С���Ա�Ʋ���Ҫԭ���Dz����������ʽ϶࣬����һ��̽���ʺ�ɫӲ�����ɡ� ����1��ȡ������ĩ���ձ��У���������3mol/LNaOH��Һ����ֽ��裬���ˣ�ϴ�ӡ�
����1��ȡ������ĩ���ձ��У���������3mol/LNaOH��Һ����ֽ��裬���ˣ�ϴ�ӡ�