��Ŀ����
��ѧ��һ����ʵ��Ϊ��������Ȼ��ѧ����ѧʵ���ڻ�ѧѧϰ�о��м�����Ҫ�����á�ij�о���ѧϰС��������ͼװ�ý���ͭ��Ũ���ᷴӦ��ʵ���о���
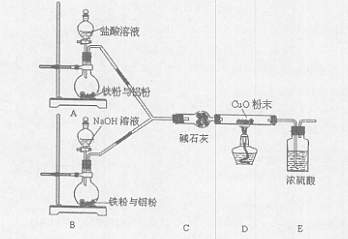
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽ ��
��2����ҪʹB ���ռ��������SO2���壨��֤ʵB�����ռ�������������װ�õ�����˳��Ϊ�� �� �� �� �� ������ĸ��ʾ����
��3��C ��ʢ�ŵ��Լ��� ��֤��B �����ռ���SO2�������� ��
��4������ƿ�г�ַ�Ӧ��ͬѧ�Ƿ���ͭ��ʣ�࣬����ⷢ������Ҳ��ʣ�ࡣ����������ʣ��ķ����� ___________________��
��5���ڲ�����Ũ�����ǰ���£�Ϊʹͭ��һ���ܽ⣬������ƿ�м��� ________������ţ����� ���� �� FeSO4 �� Fe2O3 ��KNO3

��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽ ��
��2����ҪʹB ���ռ��������SO2���壨��֤ʵB�����ռ�������������װ�õ�����˳��Ϊ�� �� �� �� �� ������ĸ��ʾ����
��3��C ��ʢ�ŵ��Լ��� ��֤��B �����ռ���SO2�������� ��
��4������ƿ�г�ַ�Ӧ��ͬѧ�Ƿ���ͭ��ʣ�࣬����ⷢ������Ҳ��ʣ�ࡣ����������ʣ��ķ����� ___________________��
��5���ڲ�����Ũ�����ǰ���£�Ϊʹͭ��һ���ܽ⣬������ƿ�м��� ________������ţ����� ���� �� FeSO4 �� Fe2O3 ��KNO3
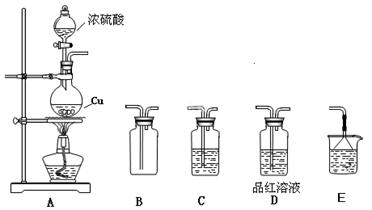
��1��Cu��2H2SO4(Ũ)��CuSO4��SO2����2H2O ��2�֣�
��2�����ã£ģ� ��2�֣� ������Ũ���ᡢD��Ʒ����Һ��ɫ ����1�֣�
��4��ȡ��Ӧ�����Һ������пƬ���������ݲ�������������ʣ�ࡣ�����������𰸾����֣� ��2�֣� ��5���ۢ� ��2�֣�
��2�����ã£ģ� ��2�֣� ������Ũ���ᡢD��Ʒ����Һ��ɫ ����1�֣�
��4��ȡ��Ӧ�����Һ������пƬ���������ݲ�������������ʣ�ࡣ�����������𰸾����֣� ��2�֣� ��5���ۢ� ��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ


 ��
��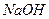 ��Һ���ⶨδ֪Ũ�ȵ������Ũ�ȣ�
��Һ���ⶨδ֪Ũ�ȵ������Ũ�ȣ�