��Ŀ����
����Ŀ���о�����CO2�ŷ���һ����Ҫ���⡣CO2��������������ɵ�̼�л����Ҫ�����·�Ӧ��
��Ӧ��CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g) ��H1����49.6 kJ/mol
CH3OH(g)��H2O(g) ��H1����49.6 kJ/mol
��Ӧ��CH3OCH3(g)��H2O(g)![]() 2CH3OH(g) ��H2����23.4 kJ/mol
2CH3OH(g) ��H2����23.4 kJ/mol
��Ӧ��2CO2(g)��6H2(g)![]() CH3OCH3(g)��3H2O(g) ��H3
CH3OCH3(g)��3H2O(g) ��H3
��1����H3��____kJ/mol��
��2�����º��������£����ܱ�������ͨ������ʵ�����CO2��H2��������ӦI������������˵����ӦI�ﵽƽ��״̬����___������ţ���
A����Ӧ��ϵ��ѹǿ���ֲ���
B�������ڵĻ��������ܶȱ��ֲ���
C��ˮ�����ж���2NA��H-O����ͬʱ������ж���3NA��H-H��
D��CH3OH��H2O��Ũ��֮�ȱ��ֲ���
��3����ӦII��ij�¶��µ�ƽ�ⳣ��Ϊ0.25�����¶��£����ܱ������м�������ʵ�����CH3OCH3(g)��H2O(g)����Ӧ��ijʱ�̲�ø����Ũ�����£�
���� | CH3OCH3(g) | H2O(g) | CH3OH(g) |
Ũ��/mol��L��1 | 1.8 | 1.8 | 0.4 |
��ʱv��___v������������������������������������Ӧ�ﵽƽ��״̬ʱ�����������CH3OH�������(CH3OH)% ��___%��
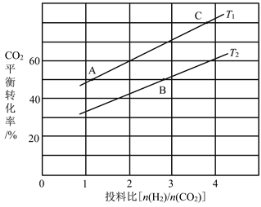
��4����ijѹǿ�£���ӦIII�ڲ�ͬ�¶ȡ���ͬͶ�ϱ�ʱ��CO2��ƽ��ת������ͼ��ʾ��T1�¶��£���6mol CO2��12mol H2����2 L���ܱ������У�5min��Ӧ�ﵽƽ��״̬����0��5min�ڵ�ƽ����Ӧ����v(CH3OCH3)��____��KA��KB��KC����֮��Ĵ�С��ϵΪ____��
��5����ѹ�½�CO2��H2�������1��3��ϣ��ڲ�ͬ���������·�����ӦI�ͷ�ӦIII������ͬ��ʱ�����CH3OH��ѡ���ԺͲ������¶ȵı仯��ͼ�����У�CH3OH��ѡ���ԣ�![]() ��100%
��100%
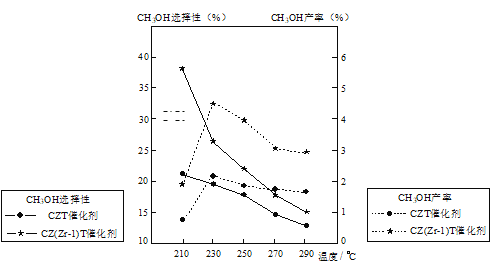
���¶ȸ���230�棬CH3OH�������¶����߶��½���ԭ����_____��
�������������ºϳɼ״��Ĺ�ҵ������____��
p>A��210�� B��230�� C������CZT D������CZ(Zr��1)T���𰸡���122.6 AC �� 20 0.18mol��L1��min1 KA��KC��KB ��ӦI����H��0�¶����ߣ�ʹCO2ת��ΪCH3OH��ƽ��ת�����½� BD
��������
��1�����ݸ�˹�����Լ��Ȼ�ѧ����ʽ���м��㣻
��2�����ݻ�ѧƽ��״̬���жϱ�־�����жϣ�
��3������Ũ���̺ͻ�ѧƽ�ⳣ���Ĵ�С��ϵ�жϷ�Ӧ���еķ�����δ֪�����û�ѧƽ�ⳣ���г�����ʽ�ⷽ�̣������������������
��4������ͼ��ȷ��CO2��ƽ��ת���ʣ���������CH3OCH3���������������û�ѧ��Ӧ���ʵļ��㹫ʽ����v(CH3OCH3)�����ݻ�ѧƽ�ⳣ��ֻ���¶��йأ��¶Ȳ��䣬��ѧƽ�ⳣ�����䣬�ó�KA��KC���ٸ��ݸ÷�ӦΪ������ȵķ�Ӧ������ƽ�������ƶ������ͼ���ж�T1��T2�Ĵ�С��ϵ���Ӷ��ó�KA��KC��KB���ߵĹ�ϵ��
��5����ӦI�ġ�H��0������ƽ�������ƶ���CO2ת��ת���ʽ��ͣ�CH3OH�����½����ٽ��ͼ���жϺϳɼ״��Ĺ�ҵ������
��1�����ݸ�˹����֪����ӦIII=��Ӧ���2-��Ӧ������ˣ���H3=��H1��2-��H2=��49.6 kJ/mol��2-23.4 kJ/mol=-122.6 kJ/mol��
�ʴ�Ϊ����122.6��
��2����Ӧ����Ϊ���º��ݣ���ӦI��CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g)Ϊ��Ӧǰ���������ʵ������ٵķ�Ӧ��
CH3OH(g)��H2O(g)Ϊ��Ӧǰ���������ʵ������ٵķ�Ӧ��
A. �÷�ӦΪ��Ӧǰ���������ʵ������ٵķ�Ӧ�����ŷ�Ӧ�Ľ����������ʵ������٣���ϵ��ѹǿ��С����˵���Ӧ��ϵ��ѹǿ���ֲ���ʱ��˵����ӦI�ﵽƽ��״̬��A����ȷ��
B. ���������غ㶨��֪�����������������䣬�����ݻ�Ҳ���䣬�����ŷ�Ӧ�Ľ��У������ڵĻ��������ܶ�ʼ�ձ��ֲ��䣬��˵��������ڵĻ��������ܶȱ��ֲ���ʱ����˵����ӦI�ﵽƽ��״��B�����
C. ��ˮ�����ж���2NA��H��O��ʱ����3NA��H��H���γɣ�������3mol����ӣ���ͬʱ������ж���3NA��H��H����������3mol����ӣ������������ʵ������ֲ��䣬��Ӧ�ﵽƽ��״̬�����ˮ�����ж���2NA��H��O����ͬʱ������ж���3NA��H��H����˵����ӦI�ﵽƽ��״��C����ȷ��
D. ��Ӧ�ﵽƽ��״̬ʱ�����ʵ����ʵ���Ũ�ȱ��ֲ��䣬��CH3OH��H2O��Ũ��֮��ʼ�յ���1:1�����CH3OH��H2O��Ũ��֮�ȱ��ֲ��䲻��˵����ӦI�ﵽƽ��״��D����ȷ��
�ʴ�Ϊ��AC��
��3���˿̷�Ӧ��Ũ����![]() ����˷�Ӧ������Ӧ������У�v��>v����
����˷�Ӧ������Ӧ������У�v��>v����
����ʼʱCH3OCH3��H2O�����ʵ�����Ϊa mol����Ӧ�ﵽƽ��״̬ʱ��CH3OCH3��ת����Ϊx mol������г�����ʽ��
CH3OCH3(g) �� H2O(g)![]() 2CH3OH(g)
2CH3OH(g)
��ʼ��mol�� a mol a mol 0
ת����mol�� x mol x mol 2x mol
ƽ�⣨mol�� (a-x)mol (a-x)mol 2x mol
��ѧƽ�ⳣ��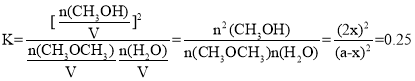 ����ã�x=0.2a������������CH3OH�����ʵ���Ϊ0.4a��������������ʵ���������Ϊ2a����˻��������CH3OH�������
����ã�x=0.2a������������CH3OH�����ʵ���Ϊ0.4a��������������ʵ���������Ϊ2a����˻��������CH3OH�������![]() ��
��
�ʴ�Ϊ������20��
��4����ͼ��֪����T1�¶��£���6 molCO2��12molH2����2 L���ܱ�������ʱ��CO2��ƽ��ת����Ϊ60%�����CO2��ת����Ϊ6 mol��60%=3.6��������CH3OCH3�����ʵ���Ϊ1.8mol��![]() ��
��
��ѧƽ�ⳣ��ֻ���¶��йأ��¶Ȳ��䣬��ѧƽ�ⳣ�����䣬��KA��KC����ͼ���֪����Ͷ�ϱ���ͬʱ��T1�¶��µ�ƽ��ת���ʽϴ÷�ӦΪ������ȵķ�Ӧ������ƽ�������ƶ�����ѧƽ�ⳣ���������T1<T2��KA��KC��KB��
�ʴ�Ϊ��0.18mol��L1��min1��KA��KC��KB��
��5���ٷ�ӦI�ġ�H��0������ͬ��ʱ����ڷ�Ӧ��230����Ӧ�ﵽƽ��״̬������ƽ�������ƶ���CO2ת��ת���ʽ��ͣ�CH3OH�����½���
�ʴ�Ϊ����ӦI�ġ�H��0�¶����ߣ�ʹCO2ת��ΪCH3OH��ƽ��ת�����½���
����ͼ���֪��230����CH3OH��ת������ߣ�����CZ(Zr��1)T��CH3OH��ѡ������ã���˺ϳɼ״��Ĺ�ҵ������230��������CZ(Zr��1)T��
�ʴ�Ϊ��BD��