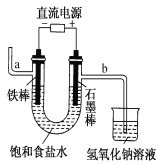
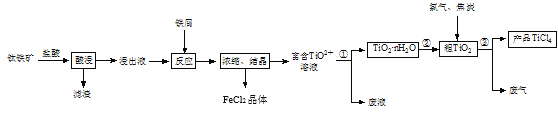
ЬтФПФкШн
ЁОЬтФПЁПФјЁЂюмЁЂюбЁЂЭЕШдЊЫиГЃгУзїжЦБИяЎРызгЕчГиЕФе§МЋВФСЯЛђИпаЇДпЛЏМСЁЃNAБэЪОАЂЗќМгЕТТоГЃЪ§ЃЌЧыЬюаДЯТСаПеАзЁЃ
ЃЈ1ЃЉЛљЬЌCoдзгЕФЕчзгХХВМЪНЮЊ___ЁЃ
ЃЈ2ЃЉФјгыCOЩњГЩЕФХфКЯЮяNi(CO)4жаЃЌвзЬсЙЉЙТЕчзгЖдЕФГЩМќдзгЪЧ___ЃЈЬюдЊЫиУћГЦЃЉЃЛ1 molNi(CO)4жаКЌгаЕФІвМќЪ§ФПЮЊ__ЃЛаДГігыCOЛЅЮЊЕШЕчзгЬхЕФвЛжжвѕРызгЕФЛЏбЇЪН_____ЁЃ
ЃЈ3ЃЉTi(BH4)2ЪЧвЛжжДЂЧтВФСЯЁЃBH4-ЕФПеМфЙЙаЭЪЧ____ЃЌBдзгЕФдгЛЏЗНЪН__ЁЃгыюбЭЌжмЦкЕФЕкЂђBзхКЭЂѓAзхСНжждЊЫижаЕквЛЕчРыФмНЯДѓЕФЪЧ___ЃЈаДдЊЫиЗћКХЃЉЃЌдвђЪЧ____ЁЃ
ЃЈ4ЃЉCuFeS2ЕФОЇАћШчЭМЫљЪОЃЌОЇАћВЮЪ§ЗжБ№ЮЊanmЁЂbnmЁЂcnmЃЛCuFeS2ЕФОЇАћжаУПИіCuдзггы___ИіSдзгЯрСЌЃЌОЇЬхУмЖШІбЃН___gЁЄcm3ЃЈСаГіМЦЫуБэДяЪНЃЉЁЃ

вдОЇАћВЮЪ§ЮЊЕЅЮЛГЄЖШНЈСЂЕФзјБъЯЕПЩвдБэЪООЇАћжаИїдзгЕФЮЛжУЃЌГЦзїдзгЗжЪ§зјБъЃЌР§ШчЭМжадзг2КЭ3ЕФзјБъЗжБ№ЮЊЃЈ0ЃЌ1ЃЌ![]() ЃЉЁЂЃЈ
ЃЉЁЂЃЈ![]() ЃЌ
ЃЌ![]() ЃЌ0ЃЉЃЌдђдзг1ЕФзјБъЮЊ___ЁЃ
ЃЌ0ЃЉЃЌдђдзг1ЕФзјБъЮЊ___ЁЃ
ЁОД№АИЁП[Ar]3d74s2 ЬМ 8NA CNЃЛђC22Ѓ е§ЫФУцЬх sp3 Zn ZnКЫЭтЕчзгХХВМЮЊШЋТњЮШЖЈНсЙЙЃЌНЯФбЪЇЕчзг 4 ![]() ЃЈ
ЃЈ![]() ЃЌ
ЃЌ![]() ЃЌ
ЃЌ![]() ЃЉ
ЃЉ
ЁОНтЮіЁП
(1)CoЪЧ27КХдЊЫиЃЌНсКЯЙЙдьдРэаДГіЕчзгХХВМЪНЃЛ
(2)NiдзгКЌгаПеЙьЕРЃЌCOгыЕЊЦјЛЅЮЊЕШЕчзгЬхЃЌЖўепЛЏбЇМќРрЫЦЃЌCOЗжзгжаCЁЂOдзгОљга1ЖдЙТЖдЕчзгЃЌНсКЯЕчИКадХаЖЯФФИідзгИќвзЬсЙЉЙТЖдЕчзгаЮГЩХфЮЛМќЃЛNi(CO)4Зжзгжага4ИіХфЮЛМќЃЌЪєгкІвМќЃЌCOжаКЌга1ИіІвМќЃЌЙЪ1ИіNi(CO)4Зжзгжага8ИіІвМќЃЛ
(3)ИљОнМлВуЕчзгЖдИіЪ§=ІвМќИіЪ§+ЙТЕчзгЖдИіЪ§МЦЫуХаЖЯBH4-жаBдзгдгЛЏРраЭКЭПеМфЙЙаЭЃЛНсКЯдзгЙьЕРжаЕчзгДІгкШЋТњЁЂШЋПеЛђАыТњЪБНЯЮШЖЈЗжЮіХаЖЯЃЛ
(4)ИљОнОљЬЏЗЈМЦЫуОЇАћжаКЌгаЕФдзгИіЪ§ЃЌдйИљОнІб=![]() МЦЫуЃЛИљОндзг2КЭ3ЕФзјБъЗжЮіХаЖЯГізјБъдЕуЃЌдйИљОнЭМЪОХаЖЯдзг1ЕФзјБъЁЃ
МЦЫуЃЛИљОндзг2КЭ3ЕФзјБъЗжЮіХаЖЯГізјБъдЕуЃЌдйИљОнЭМЪОХаЖЯдзг1ЕФзјБъЁЃ
(1)юмЮЊ27КХдЊЫиЃЌЛљЬЌюмдзгЕФКЫЭтЕчзгХХВМЪНЪЧ1s22s22p63s23p63d74s2ЛђМђаДЮЊ[Ar]3d74s2ЃЌЙЪД№АИЮЊЃК1s22s22p63s23p63d74s2Лђ[Ar]3d74s2ЃЛ
(2)NiдзгКЌгаПеЙьЕРЃЌCOгыЕЊЦјЛЅЮЊЕШЕчзгЬхЃЌЖўепЛЏбЇМќРрЫЦЃЌCOЗжзгжаCЁЂOдзгОљга1ЖдЙТЖдЕчзгЃЌгЩгкOдЊЫиЕчИКадБШЬМдЊЫиЕФДѓЃЌCдзгИќвзЬсЙЉЙТЖдЕчзгаЮГЩХфЮЛМќЃЛNi(CO)4Зжзгжага4ИіХфЮЛМќЃЌЪєгкІвМќЃЌCOжаКЌга1ИіІвМќЃЌЙЪ1ИіNi(CO)4Зжзгжага8ИіІвМќЃЌ1molNi(CO)4жаКЌгаЕФІвМќЪ§ФПЮЊ8NA(Лђ8ЁС6.02ЁС1023Лђ4.816ЁС1023)ЃЛгыCOЛЅЮЊЕШЕчзгЬхЮЂСЃга2ИідзгЁЂМлЕчзгзмЪ§ЮЊ10ЃЌвѕРызгПЩвдЪЧCN-ЛђC22ЃЃЌЙЪД№АИЮЊЃКЬМЃЛ8NA(Лђ8ЁС6.02ЁС1023Лђ4.816ЁС1023)ЃЛCN-ЛђC22ЃЃЛ
(3)ЂйBH4-жаBдзгЕФЙТЕчзгЖдЪ§=![]() =0ЃЌМлВуЕчзгЖдЪ§=0+4=4ЃЌBH4-ЕФПеМфЙЙаЭгыVSEPRФЃаЭЯрЭЌЃЌМДЮЊе§ЫФУцЬхЃЌдгЛЏЙьЕРЪ§ФПЮЊ4ЃЌBдзгВЩШЁsp3дгЛЏЃЛгыюбЭЌжмЦкЕФЕкЂђBзхКЭЂѓAзхСНжждЊЫиЮЊZnКЭGaЃЌгЩгкZnКЫЭтЕчзгХХВМЮЊШЋТњНсЙЙЃЌБШНЯЮШЖЈЃЌНЯФбЪЇЕчзгЃЌвђДЫЕквЛЕчРыФмБШGaЕФДѓЃЌЙЪД№АИЮЊЃКе§ЫФУцЬхаЮЃЛsp3дгЛЏЃЛZnЃЛZnКЫЭтЕчзгХХВМЮЊШЋТњЮШЖЈНсЙЙЃЌНЯФбЪЇЕчзгЃЛ
=0ЃЌМлВуЕчзгЖдЪ§=0+4=4ЃЌBH4-ЕФПеМфЙЙаЭгыVSEPRФЃаЭЯрЭЌЃЌМДЮЊе§ЫФУцЬхЃЌдгЛЏЙьЕРЪ§ФПЮЊ4ЃЌBдзгВЩШЁsp3дгЛЏЃЛгыюбЭЌжмЦкЕФЕкЂђBзхКЭЂѓAзхСНжждЊЫиЮЊZnКЭGaЃЌгЩгкZnКЫЭтЕчзгХХВМЮЊШЋТњНсЙЙЃЌБШНЯЮШЖЈЃЌНЯФбЪЇЕчзгЃЌвђДЫЕквЛЕчРыФмБШGaЕФДѓЃЌЙЪД№АИЮЊЃКе§ЫФУцЬхаЮЃЛsp3дгЛЏЃЛZnЃЛZnКЫЭтЕчзгХХВМЮЊШЋТњЮШЖЈНсЙЙЃЌНЯФбЪЇЕчзгЃЛ
(4)ИљОнCuFeS2ЕФОЇАћЭМЃЌОЇАћФкВПЕФУПИіCuдзггы4ИіSЯрСЌЃЌвђДЫОЇАћжаУПИіCuдзггы4ИіSдзгЯрСЌЃЛИУОЇАћжаSдзгИіЪ§=8ЁЂFeдзгИіЪ§=4ЁС![]() +4ЁС
+4ЁС![]() +2ЁС
+2ЁС![]() =4ЁЂCuдзгИіЪ§=8ЁС
=4ЁЂCuдзгИіЪ§=8ЁС![]() +4ЁС
+4ЁС![]() +1=4ЃЌдђОЇАћжЪСПЮЊ
+1=4ЃЌдђОЇАћжЪСПЮЊ![]() gЃЌОЇАћВЮЪ§ЗжБ№ЮЊanmЁЂbnmЁЂcnmЃЌдђОЇАћЬхЛ§=abc(nm3)ЃЌОЇЬхУмЖШІбЃН
gЃЌОЇАћВЮЪ§ЗжБ№ЮЊanmЁЂbnmЁЂcnmЃЌдђОЇАћЬхЛ§=abc(nm3)ЃЌОЇЬхУмЖШІбЃН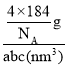 =
=![]() gЁЄcm3ЃЛЭМжадзг2КЭ3ЕФзјБъЗжБ№ЮЊ(0ЃЌ1ЃЌ
gЁЄcm3ЃЛЭМжадзг2КЭ3ЕФзјБъЗжБ№ЮЊ(0ЃЌ1ЃЌ![]() )ЁЂ(
)ЁЂ(![]() ЃЌ
ЃЌ![]() ЃЌ0)ЃЌЫЕУїЪЧвдЭМжаЕзУцзѓЧАЗНЕФЭдзгзїЮЊзјБъдЕуЕФЃЌдђдзг1ЕФзјБъЮЊ(
ЃЌ0)ЃЌЫЕУїЪЧвдЭМжаЕзУцзѓЧАЗНЕФЭдзгзїЮЊзјБъдЕуЕФЃЌдђдзг1ЕФзјБъЮЊ(![]() ЃЌ
ЃЌ![]() ЃЌ
ЃЌ![]() )ЃЌЙЪД№АИЮЊЃК4ЃЛ
)ЃЌЙЪД№АИЮЊЃК4ЃЛ![]() ЃЛ(
ЃЛ(![]() ЃЌ
ЃЌ![]() ЃЌ
ЃЌ![]() )ЃЛ
)ЃЛ

 гІгУЬтзївЕБОЯЕСаД№АИ
гІгУЬтзївЕБОЯЕСаД№АИЁОЬтФПЁПбаОПМѕЩйCO2ХХЗХЪЧвЛЯюживЊПЮЬтЁЃCO2ОДпЛЏМгЧтПЩвдЩњГЩЕЭЬМгаЛњЮяЃЌжївЊгавдЯТЗДгІЃК
ЗДгІЂёЃКCO2(g)ЃЋ3H2(g)![]() CH3OH(g)ЃЋH2O(g) ЁїH1ЃНЃ49.6 kJ/mol
CH3OH(g)ЃЋH2O(g) ЁїH1ЃНЃ49.6 kJ/mol
ЗДгІЂђЃКCH3OCH3(g)ЃЋH2O(g)![]() 2CH3OH(g) ЁїH2ЃНЃЋ23.4 kJ/mol
2CH3OH(g) ЁїH2ЃНЃЋ23.4 kJ/mol
ЗДгІЂѓЃК2CO2(g)ЃЋ6H2(g)![]() CH3OCH3(g)ЃЋ3H2O(g) ЁїH3
CH3OCH3(g)ЃЋ3H2O(g) ЁїH3
ЃЈ1ЃЉЁїH3ЃН____kJ/molЁЃ
ЃЈ2ЃЉКуЮТКуШнЬѕМўЯТЃЌдкУмБеШнЦїжаЭЈШыЕШЮяжЪЕФСПЕФCO2КЭH2ЃЌЗЂЩњЗДгІIЁЃЯТСаУшЪіФмЫЕУїЗДгІIДяЕНЦНКтзДЬЌЕФЪЧ___ЃЈЬюађКХЃЉЁЃ
AЃЎЗДгІЬхЯЕзмбЙЧПБЃГжВЛБф
BЃЎШнЦїФкЕФЛьКЯЦјЬхЕФУмЖШБЃГжВЛБф
CЃЎЫЎЗжзгжаЖЯСб2NAИіH-OМќЃЌЭЌЪБЧтЗжзгжаЖЯСб3NAИіH-HМќ
DЃЎCH3OHКЭH2OЕФХЈЖШжЎБШБЃГжВЛБф
ЃЈ3ЃЉЗДгІIIдкФГЮТЖШЯТЕФЦНКтГЃЪ§ЮЊ0.25ЃЌДЫЮТЖШЯТЃЌдкУмБеШнЦїжаМгШыЕШЮяжЪЕФСПЕФCH3OCH3(g)КЭH2O(g)ЃЌЗДгІЕНФГЪБПЬВтЕУИїзщЗжХЈЖШШчЯТЃК
ЮяжЪ | CH3OCH3(g) | H2O(g) | CH3OH(g) |
ХЈЖШ/molЁЄLЃ1 | 1.8 | 1.8 | 0.4 |
ДЫЪБvе§___vФцЃЈЬюЁАЃОЁБЁЂЁАЃМЁБЛђЁАЃНЁБЃЉЃЌЕБЗДгІДяЕНЦНКтзДЬЌЪБЃЌЛьКЯЦјЬхжаCH3OHЬхЛ§ЗжЪ§(CH3OH)% ЃН___%ЁЃ
ЃЈ4ЃЉдкФГбЙЧПЯТЃЌЗДгІIIIдкВЛЭЌЮТЖШЁЂВЛЭЌЭЖСЯБШЪБЃЌCO2ЕФЦНКтзЊЛЏТЪШчЭМЫљЪОЁЃT1ЮТЖШЯТЃЌНЋ6mol CO2КЭ12mol H2ГфШы2 LЕФУмБеШнЦїжаЃЌ5minКѓЗДгІДяЕНЦНКтзДЬЌЃЌдђ0ЁЋ5minФкЕФЦНОљЗДгІЫйТЪv(CH3OCH3)ЃН____ЃЛKAЁЂKBЁЂKCШ§епжЎМфЕФДѓаЁЙиЯЕЮЊ____ЁЃ
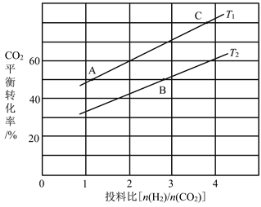
ЃЈ5ЃЉКубЙЯТНЋCO2КЭH2АДЬхЛ§БШ1ЃК3ЛьКЯЃЌдкВЛЭЌДпЛЏМСзїгУЯТЗЂЩњЗДгІIКЭЗДгІIIIЃЌдкЯрЭЌЕФЪБМфЖЮФкCH3OHЕФбЁдёадКЭВњТЪЫцЮТЖШЕФБфЛЏШчЭМЁЃЦфжаЃКCH3OHЕФбЁдёадЃН![]() ЁС100%
ЁС100%
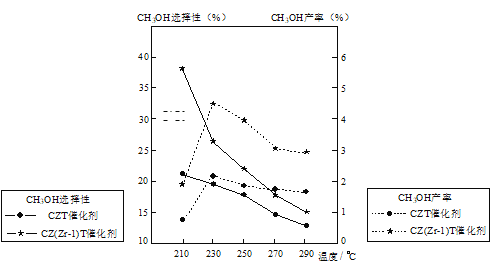
ЂйЮТЖШИпгк230ЁцЃЌCH3OHВњТЪЫцЮТЖШЩ§ИпЖјЯТНЕЕФдвђЪЧ_____ЁЃ
ЂкдкЩЯЪіЬѕМўЯТКЯГЩМзДМЕФЙЄвЕЬѕМўЪЧ____ЁЃ
p>AЃЎ210Ёц BЃЎ230Ёц CЃЎДпЛЏМСCZT DЃЎДпЛЏМСCZ(ZrЃ1)TЁОЬтФПЁПЯТСаЪЕбщВйзїВЛФмДяЕНЪЕбщФПЕФЕФЪЧ
бЁЯю | ЪЕбщФПЕФ | ЪЕбщВйзї |
A | БШНЯН№ЪєУОКЭТСЕФЛюЦУад | ЗжeЯђСНжЛЪЂгаЕШЬхЛ§ЕШХЈЖШЕФЯЁСђЫсЩеБжаМгШыДђФЅЙ§ЕФЭЌбљДѓаЁЕФУОЦЌКЭТСЦЌЃЌБШНЯЗДгІЯжЯѓ |
B | Г§ШЅMgЗлжаЛьгаЕФAl Зл | МгШызуСПЕФNaOH ШмвКЃЌЙ§ТЫЁЂЯДЕгЁЂИЩдя |
C | ЬНОПЮЌЩњЫиCЕФЛЙдад | ЯђЪЂга2 mLЛЦЩЋТШЛЏЬњШмвКЕФЪдЙмжаЕЮМгХЈЕФЮЌЩњЫиCШмвКЃЌЙлВьбеЩЋБфЛЏ |
D | ХфжЦ0.4000molЁЄL1ЕФNaOHШмвК | ГЦШЁ4.0gЙЬЬхNaOHгкЩеБжаЃЌжБНгЯђЩеБжаМгШы250mLЫЎ |
A. AB. BC. CD. D