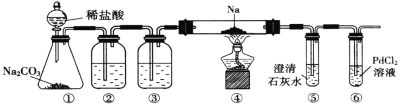
��Ŀ����
����Ŀ������þ��(Mg2B2O5��H2O����Fe2O3����)��ȡ����(H3BO3)������������¡�
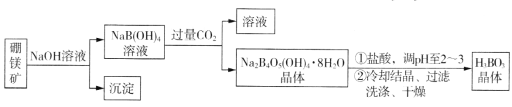
ͬ���������⣺
(1)��������Ҫ�ɷ�Ϊ____________________(�ѧʽ)��
(2)д������Na2B4O5(OH)4��8H2O�Ļ�ѧ����ʽ_________________________________��
(3)����H3BO3����ϴ�Ӹɾ��IJ�����______________________________��
(4)��֪��
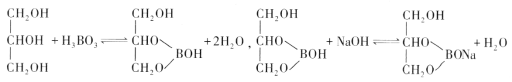
ʵ�������ô�ԭ���ⶨ������Ʒ�����������������ȷ��ȡ0.3000g��Ʒ����ƿ�У�����������ͼ���ʹ�����ܽⲢ��ȴ������1��2�η�̪��Һ��Ȼ����0.2000mol��L-1NaOH����Һ�ζ����յ㣬����NaOH��Һ22.00mL��
�ٵζ��յ������Ϊ________________________��
�ڸ�������Ʒ�Ĵ���Ϊ_________________��(����1λС��)��
(5)���NaB(OH)4��Һ�Ʊ�H3BO3�Ĺ���ԭ������ͼ��
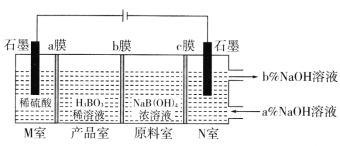
��bĤΪ________����Ĥ(������������������������)��������ÿ����1molH3BO3�������ҹ�����__________L����(��״��)��
��N���У����ںͳ���NaOH��Һ��Ũ�ȣ�a��_________b��(����>������<��)��
���𰸡�Mg(OH)2��Fe2O3 4NaB��OH��4+2CO2+3H2O=Na2B4O5��OH��48H2O��+2NaHCO3 ȡ���һ��ϴ��Һ�������Թ��У��μ������ữ����������Һ��������������˵��ϴ�Ӹɾ� ��Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ 90.9 ������ 16.8 <
��������
��þ��������������Һ��Ӧ�����˳�ȥ����Mg��OH��2��Fe2O3��NaB��OH��4��Һ��ͨ������Ķ�����̼���õ�Na2B4O5��OH��48H2O��ΪNaHCO3�����˷��룬�������������С�����ᣬ���ϸ��ֽⷴӦ��ǿ���������ԭ������������ܽ�Ƚ�С����Na2B4O5��OH��48H2O���������ᷴӦ�õ����ᣬ��ȴ�ᾧ�����ˡ�ϴ�ӡ�����õ�����(H3BO3)���塣
(1)þ���ӿ�������������þ���������������������������Ʒ�Ӧ��
(2)��Ӧ��NaB��OH��4����CO2��������Na2B4O5(OH)4��8H2O��̼�����ƣ�д����ѧ����ʽ��
(3)ȡ���һ��ϴ��Һ���������������飻
(4) �ٸ��ݷ�̪�����ɫ�����������жϣ�
�����ù�ϵʽ�����м��㣻
(5)M������������ʧ���ӣ������Ӿ���aĤ�����Ʒ�ң�aĤΪ�����ӽ���Ĥ��ԭ������B��OH��4-ͨ��bĤ�����Ʒ����M�ҽ����H+��Ӧ����H3BO3��bĤΪ�����ӽ���Ĥ��ԭ����Na+����cĤ����N�ң�cĤΪ�����ӽ���Ĥ��N�������ӵõ�����������������������Ũ��������M����������������ˮ��N�������������ƺ��������ݴ˷�����
(1) ��þ�����������Ʒ�Ӧ��þ��������������þ������Fe2O3�����������Ʒ�Ӧ����˳�������Ҫ�ɷ�ΪMg(OH)2��Fe2O3��
�𰸣�Mg(OH)2��Fe2O3
(2) ��Ӧ��NaB��OH��4����CO2��������Na2B4O5(OH)4��8H2O��̼�����ƣ���ѧ����ʽΪ4NaB��OH��4+2CO2+3H2O=Na2B4O5��OH��48H2O��+2NaHCO3��
�𰸣�4NaB��OH��4+2CO2+3H2O=Na2B4O5��OH��48H2O��+2NaHCO3
(3)����H3BO3����ϴ�Ӹɾ��IJ�����ȡ���һ��ϴ��Һ�������Թ��У��μ������ữ����������Һ��������������˵��ϴ�Ӹɾ���
�𰸣�ȡ���һ��ϴ��Һ�������Թ��У��μ������ữ����������Һ��������������˵��ϴ�Ӹɾ�
(4)�ٵζ��յ������Ϊ��Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��
�𰸣���Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ
��H3BO3��NaOH
1mol 1mol
n��H3BO3��0.2000mol/L��22.00��10-3L
��n��H3BO3��=0.0044mol
m��H3BO3��= n��H3BO3����M��H3BO3��=0.0044mol��62g/mol=0.2728g
����Ϊ![]() ��100%=90.9%
��100%=90.9%
(5)M������������ʧ�������������������Ӿ���aĤ�����Ʒ�ң�aĤΪ�����ӽ���Ĥ��ԭ������B��OH��4��ͨ��bĤ�����Ʒ����M�ҽ����H+��Ӧ����H3BO3��bĤΪ�����ӽ���Ĥ��ԭ����Na+����cĤ����N�ң�cĤΪ�����ӽ���Ĥ��N�������ӵõ�����������������������Ũ��������
�������������֪bĤΪ�����ӽ���Ĥ����ΪH++ B��OH��4��=H3BO3+H2O�����ת��1mol��������1mol H3BO3���й�ϵʽ
1mole-��1/4O2��M�ң���1mol H3BO3��1/2H2��N�ң�
������ÿ����1molH3BO3�������ҹ����ɣ�1/2+1/4��mol��22.4L/mol=16.8L����(��״��)��
�������������֪N���У����ںͳ���NaOH��Һ��Ũ�ȣ�a��<b����
�𰸣������� 16.8 <

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ����10�֣�ijͬѧ����ˮ�ʼ��վ����480 mL 0.5 mol��L��1NaOH��Һ�Ա�ʹ�á�
��1����ͬѧӦѡ��________mL������ƿ��
��2���������������ͼ��ʾ��

����ͼ����Ӧ����ͼ�е�________(��ѡ����ĸ)֮�䡣
A�������� B�������� C��������
��3����ͬѧӦ��ȡNaOH����________g��������Ϊ23.1 g���ձ�����������ƽ�ϳ�ȡ����NaOH����ʱ�����ڸ�����ѡȡ����������С________(����ĸ)��������ͼ��ѡ������ȷ��ʾ����λ�õ�ѡ��________(����ĸ)��
���� ������
a | b | c | d | e | |
�����С/g | 100 | 50 | 20 | 10 | 5 |

��4�����в�����������Һ��Ũ�ȴ�С�к�Ӱ�죿
��ת������Һ��δϴ�Ӳ��������ձ���Ũ�Ȼ�________(����ƫ��������ƫС��������Ӱ��������ͬ)
������ƿ��ԭ������������ˮ��Ũ�Ȼ�________��