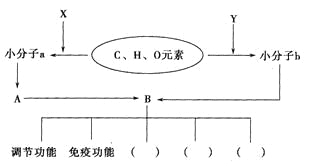
��Ŀ����
����Ŀ�����������һ�����Ƹ�Ѫѹ��ҩ����м��壬����ͨ�����·����ϳɣ�

��ش��������⣺
��1��д��C�еĹ����ŵ�����Ϊ_________________.
��2����������ķ���ʽ________________.
��3��д����Ӧ�ٵĻ�ѧ����ʽ__________________________________________����Ӧ�ڵķ�Ӧ������_____________
��4����Ӧ���м�����Լ�X�ķ���ʽΪC3H5OCl��X�Ľṹ��ʽΪ____________________.
��5����������������B��ͬ���칹������_______�֣����к˴Ź������������ֲ�ͬ��ѧ�������⣬�ҷ������Ϊ3��2��2��1��1��1����________________________(д�ṹ��ʽ)
���ܷ���������Ӧ�����ܷ���ˮ��
������FeCl3��Һ������ɫ��Ӧ
��ֻ��һ����
��6����������֪ʶ�������Ŀ���������Ϣ��д����![]() ��
�� Ϊԭ���Ʊ�
Ϊԭ���Ʊ�![]() �ĺϳ�·������ͼ(���Լ���ѡ)���ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ(���Լ���ѡ)���ϳ�·������ͼʾ�����£�![]() _______________________
_______________________
���𰸡� �ǻ����Ѽ� C15H25O3N  +CH3ONa��
+CH3ONa��![]() + NaCl ��ԭ��Ӧ
+ NaCl ��ԭ��Ӧ ![]() 23
23 





�������������л�����ƶϺͺϳɣ���1������C�Ľṹ��ʽ�����й��������ǻ����Ѽ�����2�������л���ɼ��ص㣬��������ķ���ʽΪC15H25O3N����3������A��B�ṹ��ʽ�ĶԱȣ���Ӧ��Ϊȡ����Ӧ���䷴Ӧ����ʽΪ  +CH3ONa��
+CH3ONa��![]() + NaCl������B��C�ṹ��ʽ�ĶԱȣ�B���ʻ��ϵ���ԭ��ת����Hԭ�ӣ��˷�ӦΪ��ԭ��Ӧ����4���Ա�C��D�ṹ��ʽ����Ӧ�۷�����ȡ����Ӧ�����Լ�X�ṹ��ʽΪ��
+ NaCl������B��C�ṹ��ʽ�ĶԱȣ�B���ʻ��ϵ���ԭ��ת����Hԭ�ӣ��˷�ӦΪ��ԭ��Ӧ����4���Ա�C��D�ṹ��ʽ����Ӧ�۷�����ȡ����Ӧ�����Լ�X�ṹ��ʽΪ��![]() ����5���ܷ���������Ӧ���ܷ���ˮ�⣬˵��������Ӧ�Ǽ���ij��������FeCl3��Һ������ɫ��Ӧ��˵�����з��ǻ�������������ͬ���칹�壺
����5���ܷ���������Ӧ���ܷ���ˮ�⣬˵��������Ӧ�Ǽ���ij��������FeCl3��Һ������ɫ��Ӧ��˵�����з��ǻ�������������ͬ���칹�壺 ��
�� �����ڱ�����λ����4�֣���
�����ڱ�����λ����4�֣��� (���ڱ�����λ����4��)��
(���ڱ�����λ����4��)�� �����ڱ�����λ����2������
�����ڱ�����λ����2������ ���һ��ڱ�����λ����4�֣���
���һ��ڱ�����λ����4�֣��� ���һ��ڱ�����λ����4�֣���
���һ��ڱ�����λ����4�֣��� ���һ��ڱ����ϵ�λ����2����������23�֣������ֲ�ͬ�Ļ�ѧ�������⣬�ҷ������Ϊ3��2��2��1��1��1����˽ṹ��ʽΪ��
���һ��ڱ����ϵ�λ����2����������23�֣������ֲ�ͬ�Ļ�ѧ�������⣬�ҷ������Ϊ3��2��2��1��1��1����˽ṹ��ʽΪ�� ����6��������������ϳ�·�ߣ��ó���
����6��������������ϳ�·�ߣ��ó���


 ��
��

 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д� �ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�