��Ŀ����
���£�11000C�������ܱ������з�����Ӧ��Na2SO4(s)+4H2(g)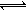 Na2S(s)+4H2O(g)
Na2S(s)+4H2O(g)
����˵���в���ȷ����
A.��ƽ�ⳣ������ʽΪ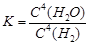
B.�����������������ѹǿ���ֲ��䣬����˵���÷�Ӧ�Ѵﵽƽ��״̬
C.��Na2SO4�������ı���ʼ����H2��Ũ�ȣ���ﵽƽ��ʱ��H2ת���ʲ���
D.����ʼʱͶ��2.84��Na2SO4��һ����H2����Ӧ�ﵽƽ��ʱ�����ڹ��干��2.264�ˣ���Na2SO4��ת����Ϊ45%
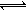 Na2S(s)+4H2O(g)
Na2S(s)+4H2O(g)����˵���в���ȷ����
A.��ƽ�ⳣ������ʽΪ
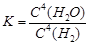
B.�����������������ѹǿ���ֲ��䣬����˵���÷�Ӧ�Ѵﵽƽ��״̬
C.��Na2SO4�������ı���ʼ����H2��Ũ�ȣ���ﵽƽ��ʱ��H2ת���ʲ���
D.����ʼʱͶ��2.84��Na2SO4��һ����H2����Ӧ�ﵽƽ��ʱ�����ڹ��干��2.264�ˣ���Na2SO4��ת����Ϊ45%
B
���ݷ���ʽ��֪����Ӧǰ����������Dz���ģ�����ѹǿʼ���Dz���ģ�ѡ��B����ȷ��AC��ȷ�����ݷ���ʽ��֪þ����142g�����ƣ�����ͼ���64g��ѡ��D�й��������0.576g���������ĵ���������1.278g�����������Ƶ�ת������1.278g��2.84g��100����45����D��ȷ����ѡB��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 N2O4(g) ��H��0���ֽ�һ����NO2��N2O4�Ļ������ͨ��һ���Ϊ1 L�ĺ����ܱ������У���Ӧ��Ũ����ʱ��仯��ϵ����ͼ��ʾ��X��Y���������У�Y��ʾN2O4Ũ����ʱ��ı仯��������˵������ȷ����
N2O4(g) ��H��0���ֽ�һ����NO2��N2O4�Ļ������ͨ��һ���Ϊ1 L�ĺ����ܱ������У���Ӧ��Ũ����ʱ��仯��ϵ����ͼ��ʾ��X��Y���������У�Y��ʾN2O4Ũ����ʱ��ı仯��������˵������ȷ����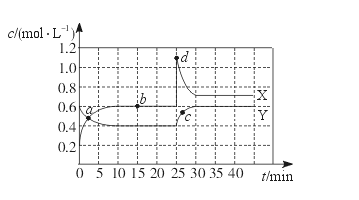
 2SO3��g���ﵽƽ��״̬��
2SO3��g���ﵽƽ��״̬��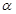 ������ϵ��ѹǿ��P���Ĺ�ϵ����ͼ1��ʾ��ƽ��״̬��A�䵽Bʱ��ƽ�ⳣ��K��A��__________K��B�����>������<����=������
������ϵ��ѹǿ��P���Ĺ�ϵ����ͼ1��ʾ��ƽ��״̬��A�䵽Bʱ��ƽ�ⳣ��K��A��__________K��B�����>������<����=������

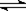 cC(g)��dD(g)����Ӧ�ﵽƽ����������ѹ����ԭ����һ�룬���ٴδﵽƽ��ʱ��D��Ũ��Ϊԭƽ���1.8��������������ȷ���� ( )
cC(g)��dD(g)����Ӧ�ﵽƽ����������ѹ����ԭ����һ�룬���ٴδﵽƽ��ʱ��D��Ũ��Ϊԭƽ���1.8��������������ȷ���� ( ) H2(g)��CO2(g)��ƽ�ⳣ�����¶ȵı仯���±���
H2(g)��CO2(g)��ƽ�ⳣ�����¶ȵı仯���±���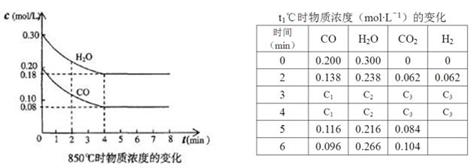
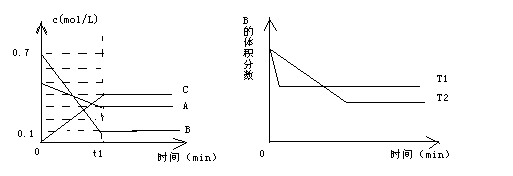
 N2 + 3H2����ij�¶��µ�ƽ�ⳣ��Ϊ0.25����ô���ڴ������£����ĺϳɷ�Ӧ1/2 N2 + 3/2 H2
N2 + 3H2����ij�¶��µ�ƽ�ⳣ��Ϊ0.25����ô���ڴ������£����ĺϳɷ�Ӧ1/2 N2 + 3/2 H2 2SO3��״̬��ʱ��ƽ�⣬��O2��ת����Ϊ�� ��
2SO3��״̬��ʱ��ƽ�⣬��O2��ת����Ϊ�� ��