��Ŀ����
����Ŀ��Ϊ��̽����������ˮ�Ļ�ԭ�ԣ�ij��ȤС��ͬѧ���������̽�����
I.̽�������Ļ�ԭ��
����ȤС��ͬѧ��������װ��(�г֣�����������)̽�������백���ķ�Ӧ������A��F�ֱ�Ϊ�����Ͱ����ķ���װ����BΪ��������������백����Ӧ��װ�á�
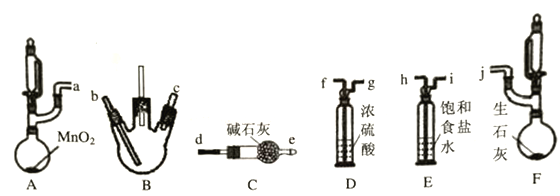
��ش�����������
��1������װ�ýӿڵ�����˳��Ϊa��h��i��f��g��___��____��___��____��j������װ��D��������____________��
(2)������������װ��B�г��ֵ�����Ϊ____________��
II.̽����ˮ�Ļ�ԭ��
����ȤС��ͬѧ̽����ͬ�����¸��������Һ�백ˮ�ķ�Ӧ��ʵ������:
ʵ�� | ���� | ���� |
�� | ȡ2mL.0.01mol/LKMnO4��Һ���Թ��У������¿���1mLŨ��ˮ�������ι�����ˮ��������Ƥ����ס�� | �����غ�ɫ����(MnO2),Լ10min����Һ�Ϻ�ɫ��dz |
�� | ȡ2mL0.01mol/LKMnO4��Һ���Թ��У������¿���1mLŨ��ˮ�������ι�1:5�����ᣬ������Ƥ����ס�� | �����غ�ɫ����(MnO2),��Һ�Ϻ�ɫ���̱�dz��Լ2min����Һ�Ϻ�ɫ��ȫ��ȥ |
�� | ȡ2mL0.1mol/LKMnO4��Һ���Թ��У������¿���ImLŨ��ˮ�������ι�����ˮ��������Ƥ����ס�� | �����غ�ɫ����(MnO2),Լ10min����Һ�Ϻ�ɫ��dz |
�� | ȡ2mL0.1mol/LKMnO4��Һ���Թ��У������¿���1mLŨ��ˮ�����˰�ι�1:5�����ᣬ������Ƥ����ס�� | �����غ�ɫ����(MnO2)����Һ�Ϻ�ɫ���̱�dz��Լ5min����Һ�Ϻ�ɫ��ȫ��ȥ |
��3��ʵ��������������ΪN2��д���÷�Ӧ�����ӷ���ʽ:_________��
��4��ʵ���٢�˵��________________��
��5��ʵ������ʵ������Ӧ����_____(����������������)��ԭ����_________��
��6��1:5��������Һ(�ܶ�Ϊ��2g��cm-3)��������������Ϊ98%��Ũ����(�ܶ�Ϊ��1g��cm-3)��
����ˮ�������1:5��ɣ����1:5��������Һ�����ʵ���Ũ��Ϊ_____mol/L��(�ú���1����2��ʽ�ӱ�ʾ)
��7����ʵ��I��II�ɵó��Ľ�����____________________��
���𰸡� c b e d �������� Bƿ����Ũ��İ��̲��������ڱ����� 2MnO4-+2NH3��H2O=2MnO2��+N2��+4H2O+2OH-
��2MnO4-+2NH3=2MnO2��+N2��+2H2O+2OH- ![]() �������������Ũ��ˮ�����������ܼӿ��������� �� ʵ�����и������Ũ�ȱ�ʵ������С ��������ˮ��-3�۵�N����ʾ��ԭ�ԣ��ܱ�ǿ�����������������������Һ����
�������������Ũ��ˮ�����������ܼӿ��������� �� ʵ�����и������Ũ�ȱ�ʵ������С ��������ˮ��-3�۵�N����ʾ��ԭ�ԣ��ܱ�ǿ�����������������������Һ����
����������1��A��F�ֱ�Ϊ�����Ͱ����ķ���װ����BΪ��������������백����Ӧ��װ�ã�װ�ýӿڵ�����˳��Ϊa��h��i��f��g��c��b��e��d��j������װ��Dװ��Ũ���ᣬ�������Ǹ���������(2)�����������������백����Ӧ�����Ȼ������백����Ӧ�����Ȼ�泥�װ��B�г��ֵ�����ΪBƿ����Ũ��İ��̲��������ڱ������3��ʵ������KMnO4��Ũ��ˮ��������������ΪN2����Ӧ�����ӷ���ʽΪ2MnO4-+2NH3��H2O=2MnO2��+N2��+4H2O+2OH-��2MnO4-+2NH3=2MnO2��+N2��+2H2O+2OH-����4��ʵ����˵���������������Ũ��ˮ��ʵ�����������ᷴӦ�Ϻ�ɫ���̱�dz��˵�����������ܼӿ��������ʣ���5��ʵ������ʵ������Ӧ��������ԭ����ʵ�����и������Ũ�ȱ�ʵ������С����6����ȡ98%��Ũ����1mL����1![]() 1mL+5mL
1mL+5mL![]() 1g/mL=VmL
1g/mL=VmL![]() ��2 �����V=
��2 �����V=![]() mL��m(H2SO4)=1mL
mL��m(H2SO4)=1mL![]() ��1g/mL
��1g/mL![]() 98%=0.98��1g ,n(H2SO4)=
98%=0.98��1g ,n(H2SO4)=![]() =0.01��1mol,����c��H2SO4��=
=0.01��1mol,����c��H2SO4��=![]() =
=![]() mol/L����7����ʵ��I��II�ɵó��Ľ����ǰ�������ˮ��-3�۵�N����ʾ��ԭ�ԣ��ܱ�ǿ�����������������������Һ������
mol/L����7����ʵ��I��II�ɵó��Ľ����ǰ�������ˮ��-3�۵�N����ʾ��ԭ�ԣ��ܱ�ǿ�����������������������Һ������

����Ŀ�������£�ijͬѧ�������백ˮ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±���
ʵ���� | ��ˮŨ��/mol��L-1 | ����Ũ��/mol��L-1 | �����ҺpH |
�� | 0.1 | 0.1 | pH��5 |
�� | c | 0.2 | pH��7 |
�� | 0.2 | 0.1 | pH��7 |
��ش�����������
��1���������û����Һc(OH-)��______ mol��L-1��
��2��������c______0.2������>����<��������������
��3���������û����Һ��������Ũ���ɴ�С��˳����_______��
��4���١������ð�ˮ�е�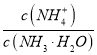 ����_______��������>����<��������������
����_______��������>����<��������������