��Ŀ����
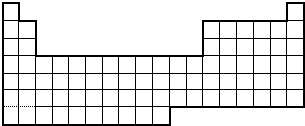
7���±�ΪԪ�����ڱ���һ���֣������г�10��Ԫ�������ڱ��е�λ�ã���Ҫ��ش��������⣮| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | �� | �� | �� | |||
| 3 | �� | �� | �� | |||||
| 4 | �� | �� |
��2������10��Ԫ���У�����������ˮ���������ǿ����KOH���ѧʽ��������������ˮ����������ǿ����HClO4���ѧʽ����
��3��H�ֱ���ܡ��ݡ��ߡ��ࡢ���γɵĻ������У����ȶ�����HF���ѧʽ����
��4���١��ࡢ������Ԫ�ص������Ӱ뾶��С�����˳���ǣ�����Ӧ�����ӷ��ű�ʾ����Na+��F-��Cl-
��5��д���ۺ͢��γɵĻ�����ĵ���ʽ��
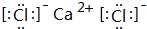 ����ѧ������Ϊ���Ӽ��������Ӽ������ۼ�������
����ѧ������Ϊ���Ӽ��������Ӽ������ۼ�������
���� ��Ԫ�������ڱ���λ�ã���֪��ΪNa����ΪK����ΪCa����ΪC����ΪN����ΪO����ΪS����ΪF����ΪCl����ΪHe��
��1��ϡ�����廯ѧ��������ã�������Խǿ��ʧȥ��������Խǿ��
��2��������Խǿ������������Ӧˮ����ļ���Խǿ��FԪ��û����ۺ����ᣬ�ʸ������������ǿ��
��3���ǽ�����Խǿ���⻯��Խ�ȶ���
��4�����Ӳ�ṹ��ͬ���˵����Խ�����Ӱ뾶ԽС�����Ӳ�Խ�����Ӱ뾶Խ��
��5���ۺ͢��γɵĻ�����ΪCaCl2���ɸ������������ӹ��ɣ�
��� �⣺��Ԫ�������ڱ���λ�ã���֪��ΪNa����ΪK����ΪCa����ΪC����ΪN����ΪO����ΪS����ΪF����ΪCl����ΪHe��
��1��ϡ������He�Ļ�ѧ��������ã�Ϊ���Т��Ԫ�أ�ͬ����������ҽ����Լ�����ͬ�������϶��½�������ǿ��������Ԫ����K�Ľ�������ǿ��Kʧȥ����������ǿ��
�ʴ�Ϊ���⣻K��
��2��������Խǿ������������Ӧˮ����ļ���Խǿ����KOH�ļ�����ǿ��FԪ��û����ۺ����ᣬ��HClO4��������ǿ��
�ʴ�Ϊ��KOH��HClO4��
��3��ͬ����������ҷǽ�������ǿ��ͬ�������϶��·ǽ����Լ�����������Ԫ����F�ķǽ�������ǿ����HF���ȶ���
�ʴ�Ϊ��HF��
��4�����Ӳ�ṹ��ͬ���˵����Խ�����Ӱ뾶ԽС�����Ӳ�Խ�����Ӱ뾶Խ�����Ӱ뾶��Na+��F-��Cl-��
�ʴ�Ϊ��Na+��F-��Cl-��
��5���ۺ͢��γɵĻ�����ΪCaCl2���ɸ������������ӹ��ɣ�����ʽΪ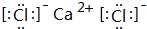 ���������Ӽ���
���������Ӽ���
�ʴ�Ϊ��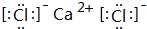 �����Ӽ���
�����Ӽ���
���� ���⿼��Ԫ�����ڱ���Ԫ���������ۺ�Ӧ�ã���Ҫѧ����������Ԫ�����ڱ���ע���Ԫ�������ɵ��������գ�

 ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д� 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�| A�� |  ������Ϊ2-�һ����� ������Ϊ2-�һ����� | |
| B�� | CH2�TCHCH3���ܷ���ȡ����Ӧ | |
| C�� | ��������Br2����һȡ�����������л��� | |
| D�� | 2-��-1��3-����ϩ������ʵ�����Br2�����ӳɷ�Ӧ�IJ�����3�� |
| A�� | ���ͼױ� | B�� | ����屽 | C�� | ˮ�ͼ��� | D�� | ˮ����ȩ |
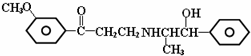
| A�� | ���Է����ӳɷ�Ӧ | B�� | ���Է�����ȥ��Ӧ | ||
| C�� | ���Է���������Ӧ | D�� | ���Է���������Ӧ |
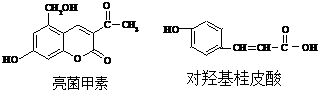
����˵����ȷ���ǣ�������
| A�� | ����1mol���������ʷֱ�������������Һ��ַ�Ӧ�����ĵ����������� | |
| B�� | ����1mol���������ʷֱ�����ˮ��ַ�Ӧ�����ĵ�������� | |
| C�� | ����1mol���������ʷֱ���������ַ�Ӧ�����ĵ������� | |
| D�� | �����л����е�����ԭ�Ӿ������ܹ�ƽ�� |
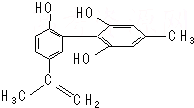 �ҹ�֧�֡����İ��ˡ�����������˶�Ա�����˷ܼ���ij���˷ܼ��Ľṹ��ʽ��ͼ��ʾ���йظ����ʵ�˵����ȷ���ǣ�������
�ҹ�֧�֡����İ��ˡ�����������˶�Ա�����˷ܼ���ij���˷ܼ��Ľṹ��ʽ��ͼ��ʾ���йظ����ʵ�˵����ȷ���ǣ�������| A�� | ��FeCl3��Һ����ɫ����Ϊ�������뱽������ͬϵ�� | |
| B�� | ����KMnO4��H+����Һ���۲���ɫ��ȥ����֤���ṹ�д���̼̼˫�� | |
| C�� | �÷����е�����ԭ���п��ܹ�ƽ�� | |
| D�� | 1 mol��������Ũ��ˮ��H2��Ӧ�������Br2��H2�ֱ�Ϊ4 mol��7 mol |
 CO2��+2SO2��+2H2O
CO2��+2SO2��+2H2O