��Ŀ����
��ͳ�ƣ�ÿ��������������������40%���ڸй���ϵ��������й���Ͼ��ع⡢��Ӱ����Ӱ��,�ڰ�Ƭ�ϵ���80%���ҽ��붨ӰҺ,��ɫƬ�ϵ�������ȫ������϶�ӰҺ�����Է϶�ӰҺ�����ĺ�����ʮ�־��˵ġ�
ij�о���ѧϰС�����ij���˾�ķ϶�ӰҺ����ʵ�鴦�����������е������塣
��һ�� ����֧�ţ�
�� ��ӰҺ(��������ƣ�Na2S2O3)����Ƭ����ֽ��û�ийⲿ�ֵ��廯����Ӧ����Ӧ����ʽΪ��AgBr(s)+ 2Na2S2O3(aq) = Na3[Ag(S2O3)2](aq) + NaBr(aq)��
�� ����������£�Na3[Ag(S2O3)2]��ת��Ϊ�����Ե�Ag2S����Ӧԭ��Ϊ��
6HCl+2Na3[Ag(S2O3)2]=6NaCl+Ag2S��+3S��+3SO2��+H2SO4+2H2O��
����Һ���������廯��������п���仹ԭ������
������ ʵ��������
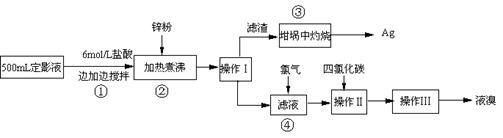
������ �Իش���������
��1����ʵ������6mol/L������250mL������ʱ������Ͳ���ձ����������⣬�����õ������У� �� ��
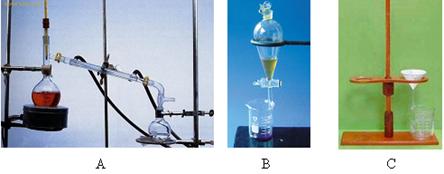
����2��ָ������ͼ�в�����������װ���ǣ�����ţ�A��B��C���� �� �� ��
��3����������ڿ��������յġ��÷�Ӧ�Ļ�ѧ����ʽ�� ��ʵ�����ʱ����������������ؼӸǺ������ȣ�������ص�Ŀ�Ŀ����ǣ� ��
��4������п�۷��������ӷ�ӦΪ�� ��
ij�о���ѧϰС�����ij���˾�ķ϶�ӰҺ����ʵ�鴦�����������е������塣
��һ�� ����֧�ţ�
�� ��ӰҺ(��������ƣ�Na2S2O3)����Ƭ����ֽ��û�ийⲿ�ֵ��廯����Ӧ����Ӧ����ʽΪ��AgBr(s)+ 2Na2S2O3(aq) = Na3[Ag(S2O3)2](aq) + NaBr(aq)��
�� ����������£�Na3[Ag(S2O3)2]��ת��Ϊ�����Ե�Ag2S����Ӧԭ��Ϊ��
6HCl+2Na3[Ag(S2O3)2]=6NaCl+Ag2S��+3S��+3SO2��+H2SO4+2H2O��
����Һ���������廯��������п���仹ԭ������
������ ʵ��������

������ �Իش���������
��1����ʵ������6mol/L������250mL������ʱ������Ͳ���ձ����������⣬�����õ������У� �� ��
����2��ָ������ͼ�в�����������װ���ǣ�����ţ�A��B��C���� �� �� ��

��3����������ڿ��������յġ��÷�Ӧ�Ļ�ѧ����ʽ�� ��ʵ�����ʱ����������������ؼӸǺ������ȣ�������ص�Ŀ�Ŀ����ǣ� ��
��4������п�۷��������ӷ�ӦΪ�� ��
��1��250mL����ƿ����ͷ�ι� ��2��C��B��A
��3��Ag2S + O2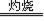 2Ag + SO2���ͷ�������ʹ������ȫ��������
2Ag + SO2���ͷ�������ʹ������ȫ��������
��4��2AgBr + Zn =" Ag " + Zn2+ + 2Br��.
��3��Ag2S + O2
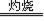 2Ag + SO2���ͷ�������ʹ������ȫ��������
2Ag + SO2���ͷ�������ʹ������ȫ����������4��2AgBr + Zn =" Ag " + Zn2+ + 2Br��.
ǰ������Ҫ�����˻���ʵ�������һ�����ʵ���Ũ����Һ�����ƣ���Ҫ�õ�����ƿ��ע��Ҫд��ͽ�ͷ�ιܡ�һ�ǹ��ˡ���ȡ��Һ���������ֻ��������ʷ��뷽����
δ֪��Ӧ�ķ���ʽ��д������ʾ�з�����������Ҫ��Ag2S����������Ag�������ڿ����з������ٲ��������IJ��롣����������ԭ���ɣ���֪������һ�ֲ������������ʽд������Ȼ�����жϳ�����ص���;��Ϊ�˷ų������Ա��������ӳ��ס�
δ֪��Ӧ�ķ���ʽ��д������ʾ�з�����������Ҫ��Ag2S����������Ag�������ڿ����з������ٲ��������IJ��롣����������ԭ���ɣ���֪������һ�ֲ������������ʽд������Ȼ�����жϳ�����ص���;��Ϊ�˷ų������Ա��������ӳ��ס�

��ϰ��ϵ�д�
�����Ŀ

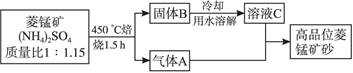
 2KMnO4+2KOH+H2��
2KMnO4+2KOH+H2��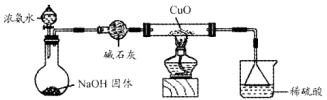
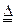 N2+3Cu+3H2O��
N2+3Cu+3H2O��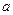 mol������ձ��е���Һ��ʹ��̪��졣�����ݡ���ѧʵ����ƻ���Ҫ������Ʊ������������Һ�ķ����������ʵ�鷽����
mol������ձ��е���Һ��ʹ��̪��졣�����ݡ���ѧʵ����ƻ���Ҫ������Ʊ������������Һ�ķ����������ʵ�鷽���� 