��Ŀ����
��12�֣�����ѧ�������ʽṹ�����ʡ�������Ԫ��A��B��C��D��AԪ�ص�ԭ�����������Ų�ʽΪms1��BԪ�ص�ԭ�Ӽ۵����Ų�ʽΪns2 np2��CԪ��λ�ڵڶ�������ԭ����p�Dz�������s�Dz����������ȣ�DԪ��ԭ�ӵ�L���p�Dz�����3��δ�ɶԵ��ӡ�
��1��CԪ��ԭ�ӻ�̬ʱ�ļ۵����Ų�ʽ ����AԪ��Ϊ�ǽ���Ԫ�أ�A��C�γɵĻ������еĹ��ۼ����� �����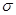 ����
����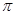 ������
������
��2����n=2ʱ��B�������̬�⻯��ķ��ӹ���Ϊ ������ԭ�ӵ��ӻ���ʽΪ ��BC2���� ���ӣ�����ԡ��Ǽ��ԡ�������n=3ʱ��B��C�γɵľ�������____ ���壻
��3����AԪ�ص�ԭ�����������Ų�Ϊ2s1��BԪ�ص�ԭ�Ӽ۵����Ų�Ϊ3s23p2��A��B��C��D����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ ����Ԫ�ط��ţ���
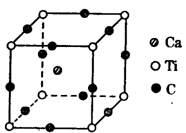
��4����ͼΪCԪ�����Ѹ�Ԫ���γɵ�ij����ṹ�е���С�ظ���Ԫ���þ�����ÿ����ԭ����Χ��������Ҿ�����ȵĸ������� �����þ���Ļ�ѧʽΪ ��
��1��CԪ��ԭ�ӻ�̬ʱ�ļ۵����Ų�ʽ ����AԪ��Ϊ�ǽ���Ԫ�أ�A��C�γɵĻ������еĹ��ۼ����� �����
 ����
����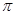 ������
��������2����n=2ʱ��B�������̬�⻯��ķ��ӹ���Ϊ ������ԭ�ӵ��ӻ���ʽΪ ��BC2���� ���ӣ�����ԡ��Ǽ��ԡ�������n=3ʱ��B��C�γɵľ�������____ ���壻
��3����AԪ�ص�ԭ�����������Ų�Ϊ2s1��BԪ�ص�ԭ�Ӽ۵����Ų�Ϊ3s23p2��A��B��C��D����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ ����Ԫ�ط��ţ���
��4����ͼΪCԪ�����Ѹ�Ԫ���γɵ�ij����ṹ�е���С�ظ���Ԫ���þ�����ÿ����ԭ����Χ��������Ҿ�����ȵĸ������� �����þ���Ļ�ѧʽΪ ��

��1��1s22s22p4��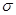 ����2���������壬sp3���Ǽ��ԣ�ԭ�Ӿ��壻��3��N��O��Si��Li����4��8��CaTiO3��
����2���������壬sp3���Ǽ��ԣ�ԭ�Ӿ��壻��3��N��O��Si��Li����4��8��CaTiO3��
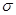 ����2���������壬sp3���Ǽ��ԣ�ԭ�Ӿ��壻��3��N��O��Si��Li����4��8��CaTiO3��
����2���������壬sp3���Ǽ��ԣ�ԭ�Ӿ��壻��3��N��O��Si��Li����4��8��CaTiO3�������������������֪��������Ԫ��A��B��C��D��AԪ�ص�ԭ�����������Ų�ʽΪms1��AԪ��λ�ڵڢ�A�壻BԪ�ص�ԭ�Ӽ۵����Ų�ʽΪns2 np2��BԪ��λ�ڵڢ�A�壻CԪ��λ�ڵڶ�������ԭ����p�Dz�������s�Dz����������ȣ���CԪ��ԭ�ӻ�̬ʱ�ļ۵����Ų�ʽΪ1s22s22p4��Ϊ��Ԫ�أ�DԪ��ԭ�ӵ�L���p�Dz�����3��δ�ɶԵ��ӣ���DԪ��ԭ�ӻ�̬ʱ�ļ۵����Ų�ʽΪ1s22s22p3��Ϊ��Ԫ�أ���1��CΪ��Ԫ��ԭ�ӻ�̬ʱ�ļ۵����Ų�ʽΪ1s22s22p4����AԪ��Ϊ�ǽ���Ԫ�أ���AΪ��Ԫ�أ�H��O�γɵĻ�����ˮ���������еĹ��ۼ���Ϊ���۵���������
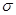 ������2����n=2ʱ��BΪ̼Ԫ�أ��������̬�⻯��CH4�ķ��ӹ���Ϊ�������壬����ԭ�ӵ��ӻ���ʽΪsp3��CO2���ڷǼ��Է��ӣ���n=3ʱ��BΪ��Ԫ�أ��������辧������ԭ�Ӿ��壻��3����AԪ�ص�ԭ�����������Ų�Ϊ2s1��AΪ�Ԫ�أ�BԪ�ص�ԭ�Ӽ۵����Ų�Ϊ3s23p2��BΪ��Ԫ�أ�A��B��C��D����Ԫ�صĵ�һ�������ɴ�С��˳��ΪN��O��Si��Li����4�����ݾ���ľ����ṹ��֪���þ�����ÿ����ԭ����Χ��������Ҿ�����ȵĸ�������8���������и���㣺1�������к���Ti��8��1/8=1��O��12��1/4=3��Ca��1���þ���Ļ�ѧʽΪCaTiO3��
������2����n=2ʱ��BΪ̼Ԫ�أ��������̬�⻯��CH4�ķ��ӹ���Ϊ�������壬����ԭ�ӵ��ӻ���ʽΪsp3��CO2���ڷǼ��Է��ӣ���n=3ʱ��BΪ��Ԫ�أ��������辧������ԭ�Ӿ��壻��3����AԪ�ص�ԭ�����������Ų�Ϊ2s1��AΪ�Ԫ�أ�BԪ�ص�ԭ�Ӽ۵����Ų�Ϊ3s23p2��BΪ��Ԫ�أ�A��B��C��D����Ԫ�صĵ�һ�������ɴ�С��˳��ΪN��O��Si��Li����4�����ݾ���ľ����ṹ��֪���þ�����ÿ����ԭ����Χ��������Ҿ�����ȵĸ�������8���������и���㣺1�������к���Ti��8��1/8=1��O��12��1/4=3��Ca��1���þ���Ļ�ѧʽΪCaTiO3��
��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ


