��Ŀ����
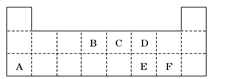
A��B��C��D��E��F�������ʵ��ת����ϵ����ͼ��ʾ����Ӧ���������ֲ���δ�г�����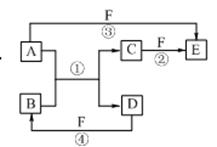
��1����A�dz����������ʣ���B��ˮ��Һ��Ӧ����C��D��D��F�����嵥�ʣ�D��F��ȼ��ʱ������ɫ���档��F����Ӧ��Ԫ�������ڱ�λ���� ����Ӧ�ڣ���ˮ��Һ�н��У������ӷ���ʽΪ ��
��2����A��DΪ������Ԫ����ɵĹ��嵥�ʣ�AΪ������DΪ�ǽ������Ңۢ�������Ӧ���к���ɫ�������ɣ���Ӧ�١��ܵĻ�ѧ����ʽ�ֱ�Ϊ
�� ���� ��
��3����A��D��F���Ƕ����ڷǽ������ʣ���A��D����Ԫ��ͬ���壬A��F����Ԫ��ͬ���ڣ�C��һ������Ѫ�쵰��ϵ��ж����壻������B�ľ��������� ������E�Ľṹʽ�� ��
��1����3���ڵ���A�壬2Fe2����Cl2=2Fe3����2Cl��
��2����2Mg+CO2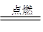 2MgO+C(�������1��)
2MgO+C(�������1��)
��C��4HNO3(Ũ) CO2����4NO2����2H2O(������д��1��)
CO2����4NO2����2H2O(�������1��)
��3��ԭ�Ӿ��� 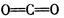
���������������1����A�dz����Ľ������ʣ���Ӧ�����û���Ӧ��D��F����̬���ʣ����ݿ�ͼ��֪��A��F����E��C��FҲ������E�����Aֻ��ΪFe��DΪH2��FΪCl2��BΪHCl��CΪFeCl2��EΪFeCl3��
��2����A��DΪ������Ԫ�ص��ʣ�������Ԫ�ص�ԭ������A��D��2��������Ԫ�ص�ԭ�Ӻ�������������D��A��2������Ӧ�����û���Ӧ�����ݿ�ͼ��֪��AΪMg��DΪC��BΪCO2��CΪMgO����֪�ۺ͢�������Ӧ�ж��к���ɫ�������ɣ�FΪHNO3��
��3����A��D��F���Ƿǽ������ʣ���Ӧ�����û���Ӧ��A��Dͬ���壬���ݿ�ͼ��֪��A��F����E��C��FҲ������E�����ֻ����SiO2��C��Ӧ����A��C��B��SiO2��C��CO��D��Si��E��CO2��F��O2��
���㣺����ͼ�ƶϡ�

 ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д� �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д� ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д� ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д�
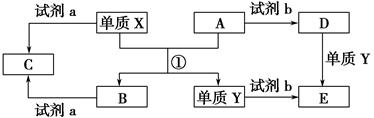
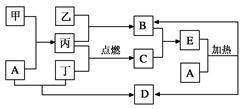
 ����Ҫ��ش��������⣺
����Ҫ��ش��������⣺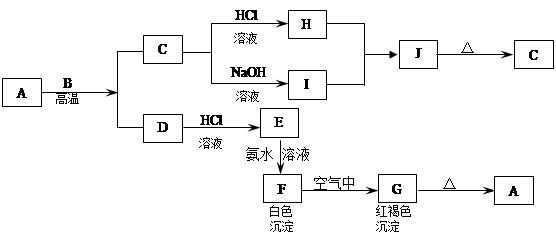

 ��1��д�������µ���X2��Z��Ӧ�����ӷ���ʽ
��1��д�������µ���X2��Z��Ӧ�����ӷ���ʽ