ЬтФПФкШн
ЁОЬтФПЁПЃЈЂёЃЉCoCl2ШмвКФмЗЂЩњШчЯТБфЛЏЃК
CoCl2![]() Co(OH)2
Co(OH)2![]() [Co(NH3)6]2+
[Co(NH3)6]2+![]() X
X![]() ОЇЬхYЃЈCoCl3ЁЄ5NH3ЃЉ
ОЇЬхYЃЈCoCl3ЁЄ5NH3ЃЉ
ЯђXШмвКжаМгШыЧПМюВЂМгШШжСЗаЬкгаАБЦјЗХГіЃЌЭЌЪБВњЩњCo2O3ГСЕэЃЛШєЯђYЕФШмвКжаМгAgNO3ШмвКЃЌгаAgClГСЕэЩњГЩЃЌТЫГіГСЕэЃЌдйЯђТЫвКжаМгШыAgNO3ШмвКЮоБфЛЏЃЌЕЋМгШШжСЗаЬкЪБгжгаAgClГСЕэЩњГЩЃЌЦфГСЕэСПЮЊЩЯДЮГСЕэСПЕФвЛАыЁЃЧыЛиД№ЯТСаЮЪЬтЃК
(1)ЗДгІЂкЕФРызгЗНГЬЪНЪЧ__________________________________________ЁЃ
(2)ЗДгІЂйЁЋЂлжаЪєгкбѕЛЏЛЙдЗДгІЕФЪЧ______ЃЌХфКЯЮяYЕФЛЏбЇЪНЮЊ______ЁЃ
ЃЈЂђЃЉвЛЖЈЬѕМўЯТЃЌНЋЕШЮяжЪЕФСПЕФCH4КЭH2O(g)ГфШы1LКуШнУмБеШнЦїЃЌЗЂЩњЗДгІCH4(g)ЃЋH2O(g) ![]() CO(g)ЃЋ3H2(g)ЃЌДяЕНЦНКтЪБВтЕУCOЕФЮяжЪЕФСПЮЊ0.10molЁЃШєЦНКтГЃЪ§KЃН27ЃЌЪдМЦЫуЃЈвЊЧѓаДГіМЦЫуЙ§ГЬЃЌМЦЫуНсЙћБЃСєСНЮЛгааЇЪ§зжЃЉЃК
CO(g)ЃЋ3H2(g)ЃЌДяЕНЦНКтЪБВтЕУCOЕФЮяжЪЕФСПЮЊ0.10molЁЃШєЦНКтГЃЪ§KЃН27ЃЌЪдМЦЫуЃЈвЊЧѓаДГіМЦЫуЙ§ГЬЃЌМЦЫуНсЙћБЃСєСНЮЛгааЇЪ§зжЃЉЃК
(1)ГѕЪММгШыШнЦїЕФМзЭщЕФЮяжЪЕФСП__________ЃЛ
(2)ЦНКтЪБCH4ЕФЬхЛ§ЗжЪ§__________ЃЛ
(3)ШєЮТЖШВЛБфЪБдйЯђЩЯЪіЦНКтЛьКЯЮяжаМгШы0.01molH2O(g)КЭ0.1molCOЃЌЦНКтЪЧЗёвЦЖЏ__________ЃП
ЁОД№АИЁПCo(OH)2+6NH3ЁЄH2O=[Co(NH3)6]2++2OH-+6H2O Ђк [Co(NH3)5Cl]Cl2 0.11mol 2.4% ВЛвЦЖЏ
ЁОНтЮіЁП
ЃЈIЃЉХфКЯЮяФкНчдкГЃЮТЯТвЛАуВЛЗЂЩњЕчРыЃЌдкМгШШЬѕМўЯТЛсЗЂЩњЕчРыЃЌОнДЫНсКЯЯрЙиЗДгІНјааХаЖЯЃЛ
ЃЈIIЃЉСаГіШ§ЖЮЪНЗжЮіИїЮяжЪЦНКтХЈЖШЃЌвРОнЦНКтГЃЪ§ЧѓГіМзЭщЕФГѕЪМЮяжЪЕФСПЃЌИљОнЫљЕУЪ§ОнЧѓГіЦНКтЪБМзЭщЕФЬхЛ§ЗжЪ§ЃЌИљОнХЈЖШьигыЦНКтГЃЪ§жЎМфЙиЯЕХаЖЯЦНКтЪЧЗёвЦЖЏЁЃ
ЃЈIЃЉЃЈ1ЃЉгЩЭМПЩжЊЃЌЗДгІЂкЮЊCo(OH)2гыNH3ЁЄH2OЗДгІЩњГЩ [Co(NH3)6]2+ЃЌЦфРызгЗДгІЗНГЬЪНЮЊЃКCo(OH)2+6NH3ЁЄH2O=[Co(NH3)6]2++2OH-+6H2OЃЌ
ЙЪД№АИЮЊЃКCo(OH)2+6NH3ЁЄH2O=[Co(NH3)6]2++2OH-+6H2OЃЛ
ЃЈ2ЃЉгЩЁАЯђXШмвКжаМгШыЧПМюВЂМгШШжСЗаЬкгаАБЦјЗХГіЃЌЭЌЪБВњЩњCo2O3ГСЕэЁБПЩжЊЃЌXжаЕФCoдЊЫиЛЏКЯМлЮЊ+3МлЃЌгЩДЫПЩжЊЃЌЗДгІЂйЁЋЂлжаЪєгкбѕЛЏЛЙдЗДгІЕФЪЧЂкЃЛ
CoCl3ЁЄ5NH3ЫЎШмжавКМгШыAgNO3ЃЌгаAgClГСЕэЩњГЩЃЌЫЕУїЭтНчРызггаCl-ЃЌЙ§ТЫКѓдйМгAgNO3ШмвКгкТЫвКжаЮоБфЛЏЃЌЕЋМгШШжСЗаЬкгаAgClГСЕэЩњГЩЃЌЫЕУїХфЬхжаКЌгаCl-ЃЌЧвЦфжЪСПЮЊЕквЛДЮГСЕэСПЕФЖўЗжжЎвЛЃЌЫЕУїЭтНчРызггаCl-гыХфЬхCl-жЎБШЮЊ2ЃК1ЃЌЙЪХфКЯЮяYЕФЛЏбЇЪНЮЊ[Co(NH3)5Cl]Cl2ЃЌ
ЙЪД№АИЮЊЃКЂкЃЛ[Co(NH3)5Cl]Cl2ЃЛ
ЃЈIIЃЉЩшМгШыCH4ЕФЮяжЪЕФСПЮЊxmolЃЌдђ
CH4(g)ЃЋH2O(g)![]() CO(g)ЃЋ3H2(g)
CO(g)ЃЋ3H2(g)
Ц№ЪМХЈЖШ(mol/L) x x 0 0
зЊЛЏХЈЖШ(mol/L) a a a 3a
ЦНКтХЈЖШ(mol/L) x-a x-a a 3a
гЩЬтПЩжЊЃЌa=0.1ЃЌгЩЦНКтГЃЪ§K=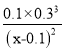 =27ЃЌНтЕУx=0.11ЃЌ
=27ЃЌНтЕУx=0.11ЃЌ
ЃЈ1ЃЉгЩЩЯЪіЗжЮіПЩжЊЃЌЦ№ЪММгШыШнЦїжаМзЭщЕФЮяжЪЕФСПЮЊ0.11molЃЌ
ЙЪД№АИЮЊЃК0.11molЃЛ
ЃЈ2ЃЉЦНКтЪБМзЭщЕФЬхЛ§ЗжЪ§=![]() ЁС100%=2.4%ЃЌ
ЁС100%=2.4%ЃЌ
ЙЪД№АИЮЊЃК2.4%ЃЛ
ЃЈ3ЃЉЯђЩЯЪіЦНКтЛьКЯЮяжаМгШы0.01molH2O(g)КЭ0.1molCOКѓЃЌc(CO)=(0.1+0.1)mol/L=0.2mol/LЃЌc(H2O)=(0.01+0.01)mol/L=0.02mol/LЃЌХЈЖШьиQC=![]() =27=KЃЌЫљвдЦНКтВЛвЦЖЏЃЌ
=27=KЃЌЫљвдЦНКтВЛвЦЖЏЃЌ
ЙЪД№АИЮЊЃКВЛвЦЖЏЁЃ

 дФЖСПьГЕЯЕСаД№АИ
дФЖСПьГЕЯЕСаД№АИЁОЬтФПЁПердкздШЛНчжаЗЧГЃЗжЩЂЃЌМИКѕУЛгаБШНЯМЏжаЕФерПѓЃЌвђДЫБЛШЫУЧГЦЮЊЁАЯЁЩЂН№ЪєЁБЁЃЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЛљЬЌердзгзюЭтВуЕчзгХХВМЭМЮЊ_______ЃЌGeдзгЕФЕчзгЗЂЩњдОЧЈЪБЛсЮќЪеЛђЗХГіВЛЭЌЕФЙтЃЌПЩгУ_______![]() ЬювЧЦїУћГЦ
ЬювЧЦїУћГЦ![]() ЩуШЁЦфдзгЙтЦзЃЌДгЖјМјЖЈGeдЊЫиЕФДцдкЁЃ
ЩуШЁЦфдзгЙтЦзЃЌДгЖјМјЖЈGeдЊЫиЕФДцдкЁЃ
ЃЈ2ЃЉердЊЫиФмаЮГЩЮоЛњЛЏКЯЮя![]() ШчерЫсФЦЃК
ШчерЫсФЦЃК![]() ЃЛЖўерЫсФЦЃК
ЃЛЖўерЫсФЦЃК![]() ЃЛЫФерЫсФЦЃК
ЃЛЫФерЫсФЦЃК![]() ЕШ
ЕШ![]() ЃЌвВФмаЮГЩРрЫЦгкЭщЬўЕФерЭщ
ЃЌвВФмаЮГЩРрЫЦгкЭщЬўЕФерЭщ![]() ЁЃ
ЁЃ
![]() жаердзгЕФдгЛЏЗНЪНЮЊ______________ЁЃ
жаердзгЕФдгЛЏЗНЪНЮЊ______________ЁЃ
![]() ергыЬМЭЌзхЃЌаджЪМАНсЙЙгавЛЖЈЕФЯрЫЦадЃЌОнДЫЭЦВт
ергыЬМЭЌзхЃЌаджЪМАНсЙЙгавЛЖЈЕФЯрЫЦадЃЌОнДЫЭЦВт![]() ЖўерЫсФЦ
ЖўерЫсФЦ![]() жаКЌгаЕФ
жаКЌгаЕФ![]() МќЕФЪ§ФПЮЊ_________ЁЃ
МќЕФЪ§ФПЮЊ_________ЁЃ
![]() жСНёУЛгаЗЂЯжnДѓгк5ЕФерЭщЃЌИљОнЯТБэЬсЙЉЕФЪ§ОнЗжЮіЦфжаЕФдвђЃК___________________ЁЃ
жСНёУЛгаЗЂЯжnДѓгк5ЕФерЭщЃЌИљОнЯТБэЬсЙЉЕФЪ§ОнЗжЮіЦфжаЕФдвђЃК___________________ЁЃ
ЛЏбЇМќ |
|
|
|
|
МќФм | 346 | 411 | 188 | 288 |
ЃЈ3ЃЉгаЛњЖрдЊьЂЫсерХфКЯЮяЪЧгЩ![]() Ђє
Ђє![]() гы
гы![]() аЮГЩЕФЃЌЦфНсЙЙШчЯТЃК
аЮГЩЕФЃЌЦфНсЙЙШчЯТЃК
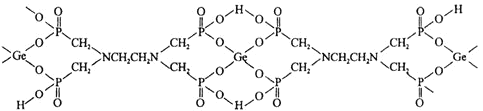
![]() ИУХфКЯЮяжаЃЌЯТСазїгУСІВЛДцдкЕФга_________
ИУХфКЯЮяжаЃЌЯТСазїгУСІВЛДцдкЕФга_________
A.МЋадМќ ![]() ЗЧМЋадМќ
ЗЧМЋадМќ ![]() Н№ЪєМќ
Н№ЪєМќ ![]() ХфЮЛМќ
ХфЮЛМќ ![]() ЧтМќ
ЧтМќ ![]() Мќ
Мќ
![]() ИУХфКЯЮяжа
ИУХфКЯЮяжа![]() Ђє
Ђє![]() ЕФХфЮЛЪ§ЪЧ_______ЃЛХфЮЛдзгЪЧ_______
ЕФХфЮЛЪ§ЪЧ_______ЃЛХфЮЛдзгЪЧ_______![]() ЬюдЊЫиЗћКХ
ЬюдЊЫиЗћКХ![]() ЁЃ
ЁЃ
![]() ЪдНтЪЭСзЫс
ЪдНтЪЭСзЫс![]() ЫсадЮЊЪВУДгыбЧЯѕЫсЯрНќЃП______________ЁЃ
ЫсадЮЊЪВУДгыбЧЯѕЫсЯрНќЃП______________ЁЃ
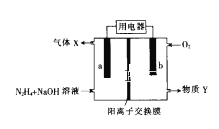
ЃЈ4ЃЉ![]() ГЃзїЮЊОќЪТЩЯЕФКьЭтжЦЕМВФСЯЃЌЦфРэЯыОЇАћШчЭМЫљЪОЁЃВтЕУОЇАћВЮЪ§
ГЃзїЮЊОќЪТЩЯЕФКьЭтжЦЕМВФСЯЃЌЦфРэЯыОЇАћШчЭМЫљЪОЁЃВтЕУОЇАћВЮЪ§![]() ЃЌ
ЃЌ![]() ЃЌИУОЇЬхЕФУмЖШЮЊ_______
ЃЌИУОЇЬхЕФУмЖШЮЊ_______![]() СаГіЫуЪНМДПЩЃЌАЂЗќМгЕТТоГЃЪ§гУ
СаГіЫуЪНМДПЩЃЌАЂЗќМгЕТТоГЃЪ§гУ![]() БэЪО
БэЪО![]() ЁЃ
ЁЃ

![]() дзгЕФЗжЪ§зјБъМДНЋОЇАћВЮЪ§aЁЂbЁЂcОљПДзїЁА1ЁБЫљЕУГіЕФШ§ЮЌПеМфзјБъЃЌвд1КХZnЮЊзјБъдЕуЃЌдђ
дзгЕФЗжЪ§зјБъМДНЋОЇАћВЮЪ§aЁЂbЁЂcОљПДзїЁА1ЁБЫљЕУГіЕФШ§ЮЌПеМфзјБъЃЌвд1КХZnЮЊзјБъдЕуЃЌдђ![]() ОЇАћЭМжаБъКХЮЊЁА2ЁБЕФPдзгЕФЗжЪ§зјБъЮЊ_______ЁЃ
ОЇАћЭМжаБъКХЮЊЁА2ЁБЕФPдзгЕФЗжЪ§зјБъЮЊ_______ЁЃ