��Ŀ����
9���״���һ����Ҫ�Ļ���ԭ�ϣ���������������Ҫ��Ӧ�ã���ҵ���ü����������ϳɼ״��ķ�Ӧ�У���CH4��g��+CO2��g��?2CO��g��+2H2��g����H1=+247.3kJ•mol-1
��CO��g��+2H2��g��?CH3OH��g����H2=-90.1kJ•mol-1
��2CO��g��+O2��g��?2CO2��g����H3=-566.0kJ•mol-1
��1����CH4��O2ֱ���Ʊ��״��������Ȼ�ѧ����ʽΪ2CH4��g��+O2��g��?2CH3OH��g����H=-251.6kJ•mol-1��
��2��ij�¶��£���4L�����ܱ�������ͨ��6mol CO2��6mol CH4��������Ӧ��i����ƽ����ϵ�и���ֵ����������Ϊ$\frac{1}{4}$������¶��¸÷�Ӧ��ƽ�ⳣ��K=1��CH4��ת����Ϊ33.3%��
��3����ҵ�Ͽ�ͨ���״��ʻ�������ȡ����������䷴Ӧ���Ȼ�ѧ����ʽΪ��CH3OH��g��+CO��g��?HCOOCH3��g��
������Ա�Ը÷�Ӧ�������о��������о�������£�

�ٴӷ�Ӧѹǿ�Լ״�ת���ʵ�Ӱ�조Ч�ʡ�������ҵ��ȡ�������Ӧѡ���ѹǿ��4.0��106Pa���3.5��106Pa����4��O��106Pa����5.0��106Pa������
��ʵ�ʹ�ҵ�����в��õ��¶���80�棬�������Ǹ���80��ʱ���¶ȶԷ�Ӧ����Ӱ���С���ҷ�Ӧ���ȣ������¶�ʱƽ�������ƶ���ת���ʽ��ͣ�
��4��þȼ�ϵ���ڿ��ƶ������豸��Դ�ͱ��õ�Դ�ȷ���Ӧ��ǰ��������ͼ3Ϊ��þ-�������Ρ�ȼ�ϵ��ԭ��ʾ��ͼ���缫Ϊþ�Ͻ�Ͳ��Ͻ�
��EΪ��ȼ�ϵ�صĸ������������������F�缫�ϵĵ缫��ӦʽΪClO-+2e-+H2O�TCl-+2OH-��
��þȼ�ϵ�ظ����������Ը�ʴ����������ʹ���������ʽ��ͣ��û�ѧ���������ԭ��Mg+2H2O�TMg��OH��2+H2����
��5����ȩ�ᣨHOOC-CHO�����л��ϳɵ���Ҫ�м��壮��ҵ���á�˫���ҳɶԵ�ⷨ��������ȩ�ᣬԭ����ͼ4��ʾ����װ��������������Ϊ���Ե缫�������Ҿ��ɲ�����ȩ�ᣬ�����Ҷ�ȩ��M�缫�IJ��ﷴӦ������ȩ�ᣮ
��N�缫�ϵĵ缫��ӦʽΪHOOC-COOH+2e-+2H+�THOOC-CHO+H2O��
������2molH+ͨ�����ӽ���Ĥ������ȫ�����˷�Ӧ�����װ�������ɵ���ȩ��Ϊ2mol��
��6���������ö��Ե缫���200mLNaCl��CuSO4�Ļ����Һ����������������ʱ��仯��ͼ5��ʾ������ͼ5����Ϣ�ش��������⣮�� ��������ѻ���ɱ�״���µ�������Һ���������ˮ�е��ܽ����Һ����ı仯��
������II �������ʾ������������ı仯��
��NaCl�����ʵ���Ũ��Ϊ0.1mol/L��CuSO4�����ʵ���Ũ��Ϊ0.1mol/L��
��t2ʱ������Һ��pHΪ1��
���� ��1�����ݸ�˹���ɽ�����2+����2+��������д�Ȼ�ѧ����ʽ��
��2������ƽ����ϵ�и���ֵ����������Ϊ$\frac{1}{4}$����������ʽ�����ƽ��ʱ����ֵĺ���������ƽ�ⳣ����ת���ʣ�
��3��������ת�������߷����жϣ���ͼ������������¶ȱ仯�����Ʒ����ش�
��4����ԭ�����ʧ���ӵ�Ϊ������������ClO-�õ������������ӣ���Mg�Ļ����Խ�ǿ����ˮ��Ӧ����������
��5����N�缫��HOOC-COOH�õ�������HOOC-CHO����2mol H+ͨ�����ӽ���Ĥ��������ת��2mol���ӣ����ݵ缫����ʽ���㣻
��6������Ĺؽ��Ƕ�ͼ��Ľ�����տ�ʼʱ����Cu2+�õ��ӣ�������ų���Cu2+��Ӧ����Һ�е�H+�ŵ磬����H2������������Һ�е�Cl-�ŵ磬��Ӧ�����Һ�е�OH-�ŵ磬���200mLһ��Ũ�ȵ�NaCl��CuSO4�����Һ����������2Cl--2e-=Cl2����4OH--4e-=O2��+2H2O����������Cu2++2e-=Cu��2H++2e-=H2�������ͼ��֪����Ϊ�������������ʱ��Ĺ�ϵ����Ϊ�������������ʱ��Ĺ�ϵ������ʱץס�����غ㣮
��� �⣺��1�����ݣ���CH4��g��+CO2��g��?2CO��g��+2H2��g����H1=+247.3kJ•mol-1
����CO��g��+2H2��g��?CH3OH��g����H2=-90.1kJ•mol-1
����2CO��g��+O2��g��?2CO2��g����H3=-566.0kJ•mol-1
�ɣ���2+����2+����2CH4��g��+O2��g��?2CH3OH��g����H���ʡ�H=2��H1+2��H2+��H3=��+247.3kJ•mol-1����2+��-90.1kJ•mol-1����2+��-566.0kJ•mol-1��=-251.6kJ•mol-1��������CH4��O2ֱ���Ʊ��״��������Ȼ�ѧ����ʽΪ2CH4��g��+O2��g��?2CH3OH��g����H=-251.6kJ•mol-1���ʴ�Ϊ��2CH4��g��+O2��g��?2CH3OH��g����H=-251.6kJ•mol-1��
��2��CH4��g��+CO2��g��?2CO��g��+2H2��g��
��ʼ��mol�� 6 6 0 0
�仯��mol�� x x 2x 2x
ƽ�⣨mol��6-x 6-x 2x 2x
ƽ����ϵ�и���ֵ����������Ϊ$\frac{1}{4}$������6-x=2x�����x=2��
����¶��¸÷�Ӧ��ƽ�ⳣ��K=$\frac{{c}^{2}��CO����{c}^{2}��{H}_{2}��}{c��C{H}_{4}����c��C{O}_{2}��}=\frac{{1}^{2}��{1}^{2}}{1��1}$=1��
CH4��ת����Ϊ$\frac{2}{6}$=33.3%���ʴ�Ϊ��1��33.3%��
��3���ٴӷ�Ӧѹǿ�Լ״�ת���ʵ�Ӱ�조Ч�ʡ�����ͼ����ת���ʱ仯������4.0��106Pa���ʴ�Ϊ��4.0��106Pa��
������ͼ������¶��ڸ���80��C�Է�Ӧ����Ӱ�첻��Ӧ�Ƿ��ȷ�Ӧ���¶ȹ��ߣ�ƽ��������У�������ת��������
�ʴ�Ϊ������80��ʱ���¶ȶԷ�Ӧ����Ӱ���С���ҷ�Ӧ���ȣ������¶�ʱƽ�������ƶ���ת���ʽ��ͣ�
��4���١�þ-�������Ρ�ȼ�ϵ����ʧ���ӵ�Ϊ��������MgΪ������������ClO-�õ������������ӣ��������ĵ缫��ӦʽΪ��ClO-+2e-+H2O�TCl-+2OH-���ʴ�Ϊ������ClO-+2e-+H2O�TCl-+2OH-��
��Mg�Ļ����Խ�ǿ����ˮ��Ӧ�����������䷴ӦΪ��Mg+2H2O�TMg��OH��2+H2�����ʴ�Ϊ��Mg+2H2O�TMg��OH��2+H2����
��5����N�缫��HOOC-COOH�õ�������HOOC-CHO����缫��ӦʽΪHOOC-COOH+2e-+2H+�THOOC-CHO+H2O���ʴ�Ϊ��HOOC-COOH+2e-+2H+�THOOC-CHO+H2O��
��2mol H+ͨ�����ӽ���Ĥ��������ת��2mol���ӣ����ݵ缫����ʽHOOC-COOH+2e-+2H+�THOOC-CHO+H2O����֪����1mol��ȩ�ᣬ��������������ȩ�������������ɵ���ȩ��Ϊ2mol���ʴ�Ϊ��2��
��6���ٵ��200mLһ��Ũ�ȵ�NaCl��CuSO4�����Һ����������2Cl--2e-=Cl2����4OH--4e-=O2��+2H2O����������Cu2++2e-=Cu��2H++2e-=H2���������������Ȳ������壬���ߢ��ʾ������������ı仯���ʴ�Ϊ����
�ڽ��ͼ��֪����Ϊ�������������ʱ��Ĺ�ϵ����Ϊ�������������ʱ��Ĺ�ϵ����ͼ��֪����������Ϊ224mL������2Cl--2e-=Cl2����֪��n��NaCl��=$\frac{0.224L}{22.4L/mol}$��2=0.02mol������c��NaCl��=$\frac{0.02mol}{0.2L}$=0.1mol/L��
��t2ʱ��������Ϊ112mL��n��O2��=$\frac{0.112L}{22.4L/mol}$=0.005mol����ת�Ƶ���Ϊ0.02mol+0.005mol��4=0.04mol��
���ݵ����غ㼰Cu2++2e-=Cu��֪��n��CuSO4��=$\frac{0.04mol}{2}$=0.02mol������c��CuSO4��=$\frac{0.02mol}{0.2L}$=0.1mol/L��
�ʴ�Ϊ��0.1mol/L��0.1mol/L��
����t2ʱ4OH--4e-=O2��+2H2O��4H+��n��H+��=0.005mol��4=0.02mol������Һ��c��H+��=$\frac{0.02mol}{0.2L}$=0.1mol/L��pH=1���ʴ�Ϊ��1��
���� ���⿼���˸�˹���ɵ�Ӧ�á��Ȼ�ѧ����ʽ�ļ�����д����ѧƽ��ļ��㡢ͼ������ж��Լ��绯ѧ��֪ʶ�ȣ�ע�����ջ���֪ʶ�����գ���Ŀ�Ѷ��еȣ�

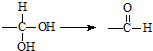 �����
���л���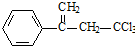 ��ѡ�������������Գ��ݼ����׳ư�ϩ����Ҫ����ˮ��������ݣ��¶Ⱥ�ʪ�ȶ�ҩЧӰ����¶ȸߡ�ʪ�ȴ�ҩЧ���ӿ죮�����йظ��л����˵������ȷ���ǣ�������
��ѡ�������������Գ��ݼ����׳ư�ϩ����Ҫ����ˮ��������ݣ��¶Ⱥ�ʪ�ȶ�ҩЧӰ����¶ȸߡ�ʪ�ȴ�ҩЧ���ӿ죮�����йظ��л����˵������ȷ���ǣ�������| A�� | ����±��������ʹ���Ը��������Һ����ˮ��ɫ | |
| B�� | �����ʼ��ж�ӳ�칹��Ҳ��˳���칹 | |
| C�� | �ڼ��������³��ˮ�⣬������������ | |
| D�� | 1mol ��������һ�������¿���4molH2�����ӳɷ�Ӧ |
CO2��g��+H2��g��?CO��g��+H2O��g�� ��ƽ�ⳣ��K���¶�t�Ĺ�ϵ���£�
| t�� | 700 | 800 | 850 | 1000 | 1200 |
| K | 2.6 | 1.7 | 1.0 | 0.9 | 0.6 |
��b�����¶�Ϊ850�棬��2L�ܱ�������ͨ��1.0mol CO2��1.0mol H2����ƽ���CO2��ת����Ϊ50%��
��2�����������õ������£�NH4+����������Ӧ��������NO3-���������������仯ʾ��ͼ���£�

��a���ڶ�����Ӧ�Ƿ��ȷ�Ӧ��ѡ����ȡ������ȡ���
��b��1molNH4+��aq��ȫ��������NO3-��aq�����Ȼ�ѧ����ʽ��NH4+ ��aq��+2O2��g���TNO3- ��aq��+2H+��aq��+H2O��l����H=-346 kJ/mol��
��3����֪��
| ��ѧʽ | ����ƽ�ⳣ�� |
| HCN | K=4.9��10-10 |
| H2CO3 | K1=4.3��10-7��K2=5.6��10-11 |
����NaCN��Һ��ͨ������CO2��������Ӧ�����ӷ���ʽΪ��CN-+CO2+H2O=HCN+HCO3-��
��4����֪��Ksp��CaCO3��=4.96��10-9����������������Ӱ�죬�ֽ�0.40mol/L��Na2CO3��Һ��0.20mol/L��CaCl2��Һ�������ϣ����Ϻ���Һ��Ca2+Ũ��Ϊ4.96��10-8mol/L��
| A�� | K+��Al3+��Cl-��NO3- | B�� | K+��Fe3+��Cl-��SiO32- | ||
| C�� | H+��Fe2+��SO42-��Br2 | D�� | K+��Ag+��NH3•H2O��NO3- |
| A�� | ���Ƿ�������ԭ��Ӧ��������������ԭ��Ӧ | |
| B�� | ������Ӧ��SO2������ֻ�൱�ڴ��� | |
| C�� | ��Ӧ����CuSO4���������� | |
| D�� | ��Ӧ����SO2������ԭ��Ӧ |
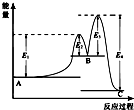 ij��Ӧ��������ӦA�TB�TC���ɣ����ķ�Ӧ����������ͼ��ʾ��E1��E2��E3��E4��ʾ��ܣ��������й�������ȷ���ǣ�������
ij��Ӧ��������ӦA�TB�TC���ɣ����ķ�Ӧ����������ͼ��ʾ��E1��E2��E3��E4��ʾ��ܣ��������й�������ȷ���ǣ�������| A�� | A�TB�ķ�Ӧ�ȡ�H=+��E2-E1��KJ/mol | B�� | ���ֻ�������C���ȶ� | ||
| C�� | C�TB �Ƿ��ȷ�Ӧ | D�� | ������Ӧ�С�H=E1-E4 |
 ��
�� 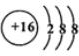 ��D��E���γ�һ������ԭ������������8���ӵķ��ӣ��÷��ӵĽṹʽΪS=C=S��D������Ԫ�ص��⻯���У��е���͵���H2S��
��D��E���γ�һ������ԭ������������8���ӵķ��ӣ��÷��ӵĽṹʽΪS=C=S��D������Ԫ�ص��⻯���У��е���͵���H2S��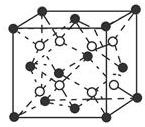 ��A��B��C��D��E����ԭ���������������Ԫ�أ�ԭ��������С��30����A�Ļ�̬ԭ��2p�ܼ���3�������ӣ�C�Ļ�̬ԭ��2p�ܼ���1�������ӣ�Eԭ���������1�������ӣ���������3���ܼ��Ҿ��������ӣ�D��Eͬ���ڣ��۵�����Ϊ2����
��A��B��C��D��E����ԭ���������������Ԫ�أ�ԭ��������С��30����A�Ļ�̬ԭ��2p�ܼ���3�������ӣ�C�Ļ�̬ԭ��2p�ܼ���1�������ӣ�Eԭ���������1�������ӣ���������3���ܼ��Ҿ��������ӣ�D��Eͬ���ڣ��۵�����Ϊ2����
 ��
�� ��
�� ��
��