��Ŀ����
1�� ��A��B��C��D��E����ԭ���������������Ԫ�أ�ԭ��������С��30����A�Ļ�̬ԭ��2p�ܼ���3�������ӣ�C�Ļ�̬ԭ��2p�ܼ���1�������ӣ�Eԭ���������1�������ӣ���������3���ܼ��Ҿ��������ӣ�D��Eͬ���ڣ��۵�����Ϊ2����
��A��B��C��D��E����ԭ���������������Ԫ�أ�ԭ��������С��30����A�Ļ�̬ԭ��2p�ܼ���3�������ӣ�C�Ļ�̬ԭ��2p�ܼ���1�������ӣ�Eԭ���������1�������ӣ���������3���ܼ��Ҿ��������ӣ�D��Eͬ���ڣ��۵�����Ϊ2������1��D��Ԫ�ط���ΪCa��A�ĵ��ʷ����Цм��ĸ���Ϊ2��
��2��BԪ�ص��⻯��ķе���ͬ��Ԫ���⻯������ߵģ�ԭ����ˮ���Ӽ�֮������������ȷ��»�����ǿ��
��3��A��B��C 3��Ԫ�صĵ�һ�������ɴ�С��˳��ΪF��N��O����Ԫ�ط��ű�ʾ����
��4��д����̬Eԭ�ӵļ۵����Ų�ʽ��3d104s1��
��5��A������⻯����ӵĿռ乹��Ϊ�����Σ�����Aԭ�ӵ��ӻ�������sp3��
��6��C��D�γɵĻ�����ľ����ṹ��ͼ��ʾ����֪������ܶ�Ϊ�� g•cm-3�������ӵ�����ΪNA�����߳�a=$\root{3}{\frac{312}{��•{N}_{A}}}$cm�����æѡ�NA�ļ���ʽ��ʾ��
���� ��A��B��C��D��E����ԭ���������������Ԫ�أ�ԭ��������С��30����A�Ļ�̬ԭ��2p�ܼ���3�������ӣ�ԭ�Ӻ�������Ų�Ϊ1s22s22p3����A��NԪ�أ�C�Ļ�̬ԭ��2p�ܼ���1�������ӣ���C��ԭ����������A����ԭ�Ӻ�������Ų�Ϊ1s22s22p5������C��FԪ�أ����ԭ����������֪B��OԪ�أ�Eԭ�Ӻ����гɵ����ӣ���������3���ܼ��Ҿ��������ӣ���ԭ������С��30����E���ڵ������ڣ����̬ԭ�ӵļ۵����Ų�ʽ[Ar]3d104s1����E��CuԪ�أ�D��Eͬ���ڣ��۵�����Ϊ2����D��CaԪ�أ��ݴ˽��
��� �⣺��A��B��C��D��E����ԭ���������������Ԫ�أ�ԭ��������С��30����A�Ļ�̬ԭ��2p�ܼ���3�������ӣ�ԭ�Ӻ�������Ų�Ϊ1s22s22p3����A��NԪ�أ�C�Ļ�̬ԭ��2p�ܼ���1�������ӣ���C��ԭ����������A����ԭ�Ӻ�������Ų�Ϊ1s22s22p5������C��FԪ�أ����ԭ����������֪B��OԪ�أ�Eԭ�Ӻ����гɵ����ӣ���������3���ܼ��Ҿ��������ӣ���ԭ������С��30����E���ڵ������ڣ����̬ԭ�ӵļ۵����Ų�ʽ[Ar]3d104s1����E��CuԪ�أ�D��Eͬ���ڣ��۵�����Ϊ2����D��CaԪ�أ�
��1��DΪCaԪ�أ�A�ĵ���Ϊ�����������ĽṹʽΪ��N��N�����Ե��������к���һ���Ҽ������м���
�ʴ�Ϊ��Ca��2��
��2��BΪ��Ԫ�أ����⻯��Ϊˮ��ˮ���Ӽ��ܴ������������ȷ��»�����ǿ��H2O�ķе���ͬ��Ԫ������ߵģ�
�ʴ�Ϊ��ˮ���Ӽ�֮��������������ȷ��»�����ǿ��
��3��ͬһ�����У�Ԫ�صĵ�һ����������ԭ��������������������ƣ���NԪ��2p�ܼ�����3�����ӣ����ڰ����ȶ�״̬�������ϵͣ���һ�����ܴ���ͬ��������Ԫ�أ��ص�һ�������ɴ�С��˳��Ϊ��F��N��O��
�ʴ�Ϊ��F��N��O��
��4��E��ͭԪ�أ����ݹ���ԭ��֪�����̬ԭ�ӵļ۵����Ų�ʽ[Ar]3d104s1���ʻ�̬Cuԭ�ӵļ۵����Ų�ʽΪ��3d104s1��
�ʴ�Ϊ��3d104s1��
��5��AΪNԪ�أ�NԪ�صļ��⻯���ǰ�����NH3�����е�ԭ�Ӽ۲���Ӷ�=3+$\frac{5-1��3}{2}$=4���Һ���һ���µ��Ӷԣ����Է��ӿռ乹���������Σ�Nԭ�Ӳ�ȡsp3�ӻ���
�ʴ�Ϊ�������Σ�sp3��
��6��F��Ca�γɵĻ�����ΪCaF2���ɾ����ṹ��֪�������а�ɫ����Ŀ=8����ɫ����Ŀ=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4����ɫ��ΪF����ɫ��ΪCa��������=4��$\frac{78g}{{N}_{A}}$=$\frac{312}{{N}_{A}}$g�����������Ϊ��a3=$\frac{\frac{312}{{N}_{A}}g}{��g•c{m}^{-3}}$=$\frac{312}{��•{N}_{A}}$cm3��
���Ըþ����߳�a=$\root{3}{\frac{312}{��•{N}_{A}}}$cm��
�ʴ�Ϊ��$\root{3}{\frac{312}{��•{N}_{A}}}$��
���� ���⿼��λ�ýṹ�����ʵ�Ӧ�ã���Ŀ�Ѷ��еȣ��漰��������Ų������ӿռ乹�͡���ѧ�����ӻ���ʽ�������ܡ���������ȣ����ǿ����ȵ㣬ע���һ�����ܵı仯���Ƽ��쳣����Ϊ�״��㣮

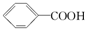 ��H2CO3��
��H2CO3��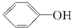 ��HCO3-���ۺϿ��Ƿ�Ӧ���ת���ʺ�ԭ�ϳɱ������أ���
��HCO3-���ۺϿ��Ƿ�Ӧ���ת���ʺ�ԭ�ϳɱ������أ��� 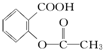 ת��Ϊ
ת��Ϊ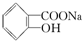 ����ѷ����ǣ�������
����ѷ����ǣ�������| A�� | ��������NaOH��Һ���Ⱥ���ͨ������CO2 | |
| B�� | ��������NaOH��Һ���Ⱥ��ټ�������H2SO4 | |
| C�� | ��ϡH2SO4���Ⱥ���������NaOH��Һ | |
| D�� | ��ϡH2SO4���Ⱥ���������Na2CO3��Һ |
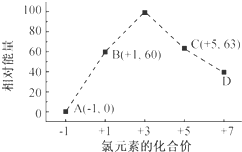
| A�� | ��FeI2����Һ�У�ͨ������Cl2��2Fe2++2I-+2Cl2�TI2+2Fe3++4Cl- | |
| B�� | Fe��OH��3��������Fe��OH��3+3H+�TFe3++3H2O | |
| C�� | ��ˮ������ͨ�������CO2��SiO32-+2CO2+2H2O�TH2SiO3��+2HCO3- | |
| D�� | ����������KIO3��Һ��KI��Һ������Ӧ����I2��IO3-+5I-+3H2O�T3I2+6OH- |
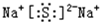 ��BA2�Ľṹʽ�ǣ�S=C=S��
��BA2�Ľṹʽ�ǣ�S=C=S��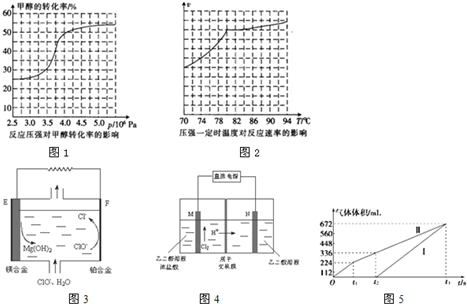
 Ԫ�����ڱ��е� VIIA��Ԫ�صĵ��ʼ��仯�������;�㷺��
Ԫ�����ڱ��е� VIIA��Ԫ�صĵ��ʼ��仯�������;�㷺��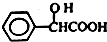 ����ҵ�ϱ������������A�������²���ϳɵõ���
����ҵ�ϱ������������A�������²���ϳɵõ���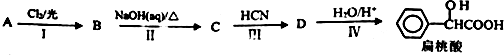
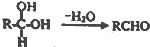
 ���������к��������������ǻ����Ȼ���
���������к��������������ǻ����Ȼ��� ��
��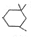 ����Ũ���������·�Ӧ���ɣ���д����Ӧ�Ļ�ѧ����ʽ
����Ũ���������·�Ӧ���ɣ���д����Ӧ�Ļ�ѧ����ʽ
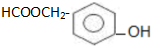 ��
��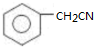 ��Ϊԭ�Ϻϳɱ������ͬ���칹��-���ǻ������ᣨ
��Ϊԭ�Ϻϳɱ������ͬ���칹��-���ǻ������ᣨ ���ĺ���·�ߣ�
���ĺ���·�ߣ�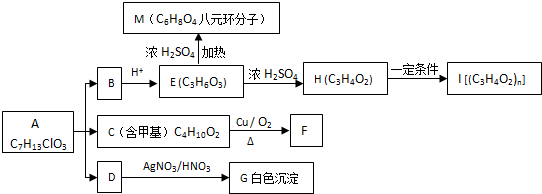
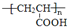
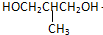 +O2$��_{��}^{Cu}$
+O2$��_{��}^{Cu}$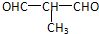 +2H2O����E��M��2HOCH2CH2COOH $��_{��}^{ŨH_{2}SO_{4}}$
+2H2O����E��M��2HOCH2CH2COOH $��_{��}^{ŨH_{2}SO_{4}}$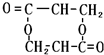 +2H2O��
+2H2O��