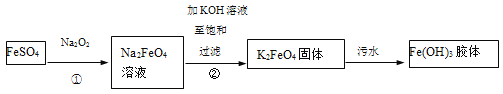
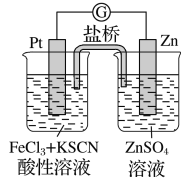
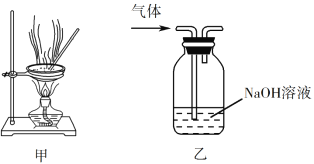
��Ŀ����
����Ŀ��25��ʱ��������ĵ���ƽ�ⳣ�����±���ʾ��
��ѧʽ |
|
|
|
���� | ���� | ������ | ������ |
����ƽ�ⳣ�� |
|
|
|
�ش��������⣺
(1)pH��ȵ�![]() ��Һ��
��Һ��![]() ��Һ��
��Һ��![]() ��Һ�У����ʵ���Ũ����С����____________���ѧʽ����
��Һ�У����ʵ���Ũ����С����____________���ѧʽ����
(2)��֪![]() Ϊ���Σ����ݱ������ݣ�
Ϊ���Σ����ݱ������ݣ�![]() �ĵڶ�������ƽ�ⳣ������ʽ
�ĵڶ�������ƽ�ⳣ������ʽ![]() ___________��25��ʱ��5.6mol��L-1
___________��25��ʱ��5.6mol��L-1![]() ��Һ��pH=____________��
��Һ��pH=____________��
(3)�������Ϊ10mL��![]() ��Ϊ
��Ϊ![]() mol��L-1��
mol��L-1��![]() ��Һ��һԪ��
��Һ��һԪ��![]() ��Һ�У��ֱ��ˮϡ����1000mL��ϡ������
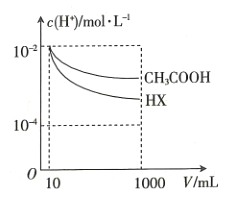
��Һ�У��ֱ��ˮϡ����1000mL��ϡ������![]() �ı仯��ͼ��ʾ����HX�ĵ���ƽ�ⳣ��____________��������������С����������������
�ı仯��ͼ��ʾ����HX�ĵ���ƽ�ⳣ��____________��������������С����������������![]() �ĵ���ƽ�ⳣ����������______________________________��
�ĵ���ƽ�ⳣ����������______________________________��
(4)�����£���0.05mol��L-1�İ�ˮ��μ���10mL0.1mol��L-1![]() ��Һ������Һ�����ԣ���ʱ��Һ�и�����Ũ�ȴ�С��ϵΪ_________________��
��Һ������Һ�����ԣ���ʱ��Һ�и�����Ũ�ȴ�С��ϵΪ_________________��
���𰸡�![]()
 10 ����
10 ���� ![]() ��ͬ��
��ͬ��![]() ��Һ��
��Һ��![]() ��Һϡ����ͬ�ı�����
��Һϡ����ͬ�ı�����![]() ��Һ��
��Һ��![]() �仯����
�仯���� ![]()
��������
(1)��ĵ���ƽ�ⳣ��ԽС������Խ��������ͬpHֵʱ�����Ũ��Խ���ݱ������ݿ�֪Ka1(H3PO3) >Ka(CHCOOH)> Ka(HClO)>������pH��ȵ�������ҺH3PO3��Ũ����С��
(2)Na2HPO3Ϊ���Σ���H3PO3Ϊ��Ԫ���ᣬ��ڶ������뷽��ʽΪH2PO3-![]() HPO32-+H+�������ƽ�ⳣ������ʽΪ
HPO32-+H+�������ƽ�ⳣ������ʽΪ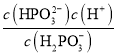 ��Na2HPO3��Һ�д�����ˮ��ƽ��HPO32-+H2O
��Na2HPO3��Һ�д�����ˮ��ƽ��HPO32-+H2O![]() H2PO3-+OH-����c(OH-)=a������ˮ��ƽ�ⳣ��Ϊ
H2PO3-+OH-����c(OH-)=a������ˮ��ƽ�ⳣ��Ϊ �����aԼΪ10-4mol/L������Һ��c(H+)=10-10mol/L��pH=10��
�����aԼΪ10-4mol/L������Һ��c(H+)=10-10mol/L��pH=10��
(3)����Խ��ϡ��ʱpH��c(H+)�仯Խ������ͼc(H+)��ͬ�Ĵ�����Һ��HX��Һϡ����ͬ�ı�����HX��Һ��c(H+)�仯��������HX�ĵ���ƽ�ⳣ�����ڴ���ĵ���ƽ�ⳣ����
(4)��Һ�д��ڵ���غ㣺c(NH4+)+ c(H+)=c(CH3COO-)+ c(OH-)������Һ����������c(H+)=c(OH-)������c(NH4+)=c(CH3COO-)��ˮ�ĵ���̶Ƚ�С��������Ũ�ȴ�С˳��Ϊc(NH4+)=c(CH3COO-)��c(H+)=c(OH-)��