��Ŀ����
�±���Ԫ�����ڱ�����Ԫ�ص�һ���֣�������Ԫ��X����������ϼ��ǣ�5��Y�ĵ���
���ڿ�����ȼ�ա�
| W | X | Y |
| | | Z |
��ش��������⣺
(1)Z��Ԫ�ط����� ��д��Z��ԭ�ӽṹʾ��ͼ�� ��
(2)W����������ﲻ����ˮ�����������ռ���Һ���÷�Ӧ�����ӷ���ʽΪ ��
(3)̽��ͬ����Ԫ�����ʵ�һЩ��ͬ���ɣ���ѧϰ��ѧ����Ҫ����֮һ�����±����г���H2ZO3���ֲ�ͬ��ѧ���ʵ��Ʋ⣬������д����Ӧ�Ļ�ѧ����ʽ(��ѧ����ʽ��Z��Ԫ�ط��ű�ʾ)
| ��� | �����Ʋ� | ��ѧ����ʽ |
| ʾ�� | ������ | H2ZO3��4HI=Z����2I2��3H2O |
| 1 | | |
| 2 | | |
(4)��C��O��Y����Ԫ����ɵĻ�����COY�У�����ԭ�ӵ�����㶼����8���ӽṹ��д���û�����ĵ���ʽ�� ��
(1)Se 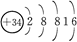
(2)SiO2��2OH��=SiO32-��H2O
(3)1 ��ԭ�� 2H2SeO3��O2=2H2SeO4(��Br2��H2SeO3��H2O=H2SeO4��2HBr�Ⱥ�����) 2 ���� H2SeO3��2NaOH=Na2SeO3��2H2O(������������)
(4) 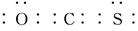
����

��ϰ��ϵ�д�
 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
�����Ŀ
��16�֣�X��Y��Z��W�dz��������ֶ�����Ԫ�أ���ԭ���������������������Ϣ���±���
| Ԫ�� | �����Ϣ |
| X | X�Ļ�̬ԭ�����������Ų�ʽΪnsnnpn |
| Y | Y�ǿ����к�����ߵ�Ԫ�� |
| Z | Z�ǵؿ��к�����ߵĽ���Ԫ�� |
| W | W�ĵ����dz����İ뵼����� |
��2��Z�ĵ�����W�ĵ�������۵�ϸߵ��� ��д��ѧʽ����Z��Wԭ�Ӱ뾶�ϴ���� ��д��ѧʽ��
��3��X��һ��������XO2�����ЦҼ���Ǽ���Ŀ֮��Ϊ
��4��X�ĵ�һ�����ܱ�W�� �����С����
��5��д��X�ĵ�����Y�����������ˮ�����Ũ��Һ�ڼ��ȵ������·�Ӧ�Ļ�ѧ����ʽ��
��6����֪2.7gZ����������Y���ʷ�Ӧ���ų�31.8kJ����������д���˷�Ӧ���Ȼ�ѧ����ʽ�� ��
������������Ԫ�صĵ��������ݣ���λ��kJ��mol��1�����ش��������⡣
| Ԫ�ش��� | I1 | I2 | I3 | I4 |
| Q | 2 080 | 4 000 | 6 100 | 9 400 |
| R | 500 | 4 600 | 6 900 | 9 500 |
| S | 740 | 1 500 | 7 700 | 10 500 |
| T | 580 | 1 800 | 2 700 | 11 600 |
| U | 420 | 3 100 | 4 400 | 5 900 |
��1�������ڱ��У�����ܴ���ͬһ����ǣ� ����
A��Q��R
B��S��T
C��T��U
D��R��T
E��R��U
��2��������ǵ������Ȼ�������缫��Ӧʽ�������ȷ���ǣ� ����
A��Q2����2e����Q
B��R2����2e����R
C��S3����3e����S
D��T3����3e����T
E��U2����2e����U
��3�����ǵ��Ȼ���Ļ�ѧʽ���������ȷ���ǣ� ����
A��QCl2
B��RCl
C��SCl3
D��TCl
E��UCl4
��4������Ԫ���У���ѧ���ʺ�������������QԪ�ص��ǣ� ����
A����1s22s22p1��
B���루1s22s2��
C���1s22s1
D���⣨1s1��
E������1s2��
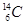 ��
��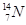 ��
�� ��
�� ��
��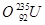 ��
��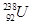 ��
�У�