��Ŀ����
��16�֣�X��Y��Z��W�dz��������ֶ�����Ԫ�أ���ԭ���������������������Ϣ���±���
| Ԫ�� | �����Ϣ |
| X | X�Ļ�̬ԭ�����������Ų�ʽΪnsnnpn |
| Y | Y�ǿ����к�����ߵ�Ԫ�� |
| Z | Z�ǵؿ��к�����ߵĽ���Ԫ�� |
| W | W�ĵ����dz����İ뵼����� |
��2��Z�ĵ�����W�ĵ�������۵�ϸߵ��� ��д��ѧʽ����Z��Wԭ�Ӱ뾶�ϴ���� ��д��ѧʽ��
��3��X��һ��������XO2�����ЦҼ���Ǽ���Ŀ֮��Ϊ
��4��X�ĵ�һ�����ܱ�W�� �����С����
��5��д��X�ĵ�����Y�����������ˮ�����Ũ��Һ�ڼ��ȵ������·�Ӧ�Ļ�ѧ����ʽ��
��6����֪2.7gZ����������Y���ʷ�Ӧ���ų�31.8kJ����������д���˷�Ӧ���Ȼ�ѧ����ʽ�� ��
��1�������ڢ�A�壬NH3����2��Si��Al����3��1:1����4����
��5��C+4HNO3(Ũ)  CO2����4NO2����2H2O����6��2Al(s)+N2(g)=2AlN(s) ?H= ��636KJ/mol
CO2����4NO2����2H2O����6��2Al(s)+N2(g)=2AlN(s) ?H= ��636KJ/mol
����

��ϰ��ϵ�д�
 ÿ�α���ϵ�д�
ÿ�α���ϵ�д� ��ѧ����ϵ�д�
��ѧ����ϵ�д�
�����Ŀ
��15�֣�A��B��C��D��EΪԭ���������������Ԫ�أ�����ֻ��E�����ڶ����ڣ������Ϣ���±���
| Ԫ�� | A | B | C | D | E |
| ��� ��Ϣ | �������������۴�����Ϊ2 | ��Ԫ��C���γ����Ӹ�����Ϊ2��1��1��1�Ļ����� | ����������ͨ��������ú���� | DԪ�ؿ��γ��������������һ�����γ��������Ҫ�ɷ� | �䵥������;��㷺�Ľ���������ȱ�ٸ�Ԫ����ƶѪ֢ |
��1��C��Ԫ�����ڱ��е�λ���� ��
��2��B��DԪ�ض�Ӧ����Է���������С���⻯�����ȷֽ������¶�B D�����������������������
��3������E3+���ӵķ����� ��
��4������D��������������Լ��� ������һ�֣������³�ѹ��DO2��һ����̼��Ӧ���� 1.6g D��������һ����������ų�14.86kJ��������д���˷�Ӧ���Ȼ�ѧ����ʽ ��
��5��0.1mol��L-1C2D��Һ�и�������Ũ�ȴӴ�С��˳���� ��
��6��AO2��O2������NaAO3������ȼ�ϵ�أ���ԭ����ͼ��ʾ���õ����ʹ�ù��̵缫������������Y��д���缫��ķ�Ӧʽ ��

(12��) ���Ʒ��ǿ�ѧѧϰ����Ҫ����֮һ
���������ƽ�����ȷ����
| | ��ȶ��� | ���� |
| A | Cl2+H2O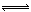 HCl+HClO HCl+HClO | I2+H2O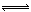 HI+HIO HI+HIO |
| B | C+2CuO ="==" 2Cu+CO2�������������ȣ� | C+SiO2 ="==" Si+ CO2�������������ȣ� |
| C | Na2O+H2O ="=" 2NaOH | CuO+H2O ="=" Cu(OH)2 |
| D | Ca(ClO)2+CO2+H2O="=" CaCO3��+2HClO | Ca(ClO)2+SO2+H2O="=" CaSO3��+2HClO |
| Ԫ�� | 8O | 16S | 34Se | 52Te |
| �����۵�(��) | ��218.4 | 113 | | 450 |
| ���ʷе�(��) | ��183 | 444.6 | 685 | 1390 |
| ��Ҫ���ϼ� | ��2 | ��2��+4��+6 | ��2��+4��+6 | |
| ԭ�Ӱ뾶 | ������ | |||
| ������H2��Ӧ��� | ��ȼʱ���� | ���Ȼ��� | �����ѻ��� | ����ֱ�ӻ��� |
�� �ڵĻ��ϼۿ��ܵķ�Χ_______ ��
�� �������ڵ��⻯��ˮ��Һ��������ǿ������˳���� (�ѧʽ)��
�� �������н�ǿ��________(������ԡ���ԭ�ԡ�)����˷��ڿ����г��ڱ����ױ��ʣ�����ܷ����Ļ�ѧ����ʽΪ___________________________________��
��24�֣��±���Ԫ�����ڱ���һ���֣���Ա��Т١�����Ԫ�أ���д���пհף�
| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
| 2 | | | | �� | �� | �� | �� | |
| 3 | �� | �� | �� | | | | �� | |
| 4 | �� | �� | | | | | | |
��2��������������ˮ�����У�������ǿ�Ļ�����Ļ�ѧʽ�� ��������ǿ�Ļ�����Ļ�ѧʽ�� ��
��3������������������������Ԫ���ڵ� �壻д������������������������Һ��Ӧ�����ӷ���ʽ ��
��4���Ӣݵ����Ԫ���У� ԭ�Ӱ뾶�����Ԫ�ط��ţ���
��5��Ԫ�آ�����γɵĻ��������� ���� �����ۡ������ӡ��������
��6����Ҫ�ȽϢݱȢĽ�����ǿ��������ʵ�鷽�����е��� ��
A�������ʢ����ڢ�����Һ�У�����ݲ����û������ʢޣ�˵���ݵĽ�������
B���ȽϢݺ͢�����������Ӧˮ�����ˮ���ԣ�ǰ�߱Ⱥ����ܽ�ȴ�ǰ�߽�����ǿ
C�����ݡ��ĵ��ʷֱ�Ͷ�뵽ˮ�У��۲쵽����ˮ��Ӧ�����ң�˵���ݵĽ�����ǿ
D�����ݡ��ĵ��ʷֱ���O2��ȼ�գ�ǰ�ߵõ����������ɫ�Ⱥ��ߵõ����������ɫ���ǰ�߽�����ǿ
�±���Ԫ�����ڱ���һ����, ��Ա��еĢ١�����Ԫ��,��д���пհ�(��д��Ų��÷�)��
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | | �� | | �� | �� | �� |
| 4 | �� | | | | | | �� | |
��1������ЩԪ����,��ѧ��������õ��ǣ� ������Ԫ�ط��ţ�
��2��������������ˮ�����У�������ǿ�Ļ�����Ļ�ѧʽ�� ��������ǿ�Ļ�����Ļ�ѧʽ�ǣ� ��
��3���ȽϢ���ݵ�����������Ӧ��ˮ��� ������ǿ���ѧʽ������ͨ�� ˵����д��Ӧ�Ļ�ѧ����ʽ����
��4��ʵ������ȡ�ڵ��⻯��Ļ�ѧ����ʽ ��
�ڵ��⻯����ڵ�����������ˮ���ﷴӦ���õIJ��ﻯѧʽΪ ��
��5���ڿ����γɶ������������һ���Ǻ���ɫ���壬���÷���ʽ˵�������岻�˲�����ˮ���ռ���ԭ�� ���û�ѧ����ʽ��ʾ����
��6���ȽϢ�����⻯� ���ȶ����ѧʽ����
��7��д���ܵĵ�����ˮ��Ӧ�����ӷ���ʽ ��
��8��д����Ԫ�ص����ӽṹʾ��ͼ ,�����Ӱ뾶 S2-�������������д����Ԫ�������ڱ���λ�� ��
�±���Ԫ�����ڱ�����Ԫ�ص�һ���֣�������Ԫ��X����������ϼ��ǣ�5��Y�ĵ���
���ڿ�����ȼ�ա�
| W | X | Y |
| | | Z |
��ش��������⣺
(1)Z��Ԫ�ط����� ��д��Z��ԭ�ӽṹʾ��ͼ�� ��
(2)W����������ﲻ����ˮ�����������ռ���Һ���÷�Ӧ�����ӷ���ʽΪ ��
(3)̽��ͬ����Ԫ�����ʵ�һЩ��ͬ���ɣ���ѧϰ��ѧ����Ҫ����֮һ�����±����г���H2ZO3���ֲ�ͬ��ѧ���ʵ��Ʋ⣬������д����Ӧ�Ļ�ѧ����ʽ(��ѧ����ʽ��Z��Ԫ�ط��ű�ʾ)
| ��� | �����Ʋ� | ��ѧ����ʽ |
| ʾ�� | ������ | H2ZO3��4HI=Z����2I2��3H2O |
| 1 | | |
| 2 | | |
(4)��C��O��Y����Ԫ����ɵĻ�����COY�У�����ԭ�ӵ�����㶼����8���ӽṹ��д���û�����ĵ���ʽ�� ��
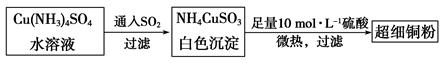
 �Ŀռ乹��Ϊ_____________��
�Ŀռ乹��Ϊ_____________��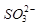 ��������ԭ�ӵ��ӻ���ʽΪ ��
��������ԭ�ӵ��ӻ���ʽΪ ��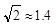 ,��ʽ������������
,��ʽ������������