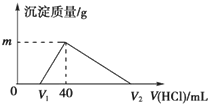
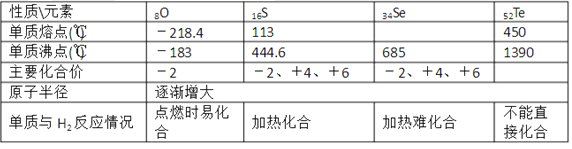
��Ŀ����
����Ŀ��373Kʱ��0.24 mol��ɫ��N2O4����ͨ�����500 mL���ܱ������У�������Ӧ��N2O4![]() 2NO2���������������ֺ���ɫ����Ӧ���е�2sʱ���������NO2�����ʵ���Ϊ0.04 mol����Ӧ���е�60 sʱ�ﵽƽ�⣬��ʱ������N2O4��NO2�����ʵ�����ȡ�����㣺����С��Ҫ��д������̣�
2NO2���������������ֺ���ɫ����Ӧ���е�2sʱ���������NO2�����ʵ���Ϊ0.04 mol����Ӧ���е�60 sʱ�ﵽƽ�⣬��ʱ������N2O4��NO2�����ʵ�����ȡ�����㣺����С��Ҫ��д������̣�
�ٿ�ʼ2 s����N2O4��ʾ�Ļ�ѧ��Ӧ����______��
�ڴﵽƽ��ʱ��ϵ��ѹǿ�뿪ʼʱ��ϵ��ѹǿ֮��______��
��N2O4��ƽ��ת����______��
���𰸡�0.02mol/(Ls) 4��3 33.3����1/3
��������
�ٸ���v=![]() ����NO2��ʾ�Ļ�ѧ��Ӧ���ʣ��ٸ�������֮�ȵ��ڼ�����֮�ȣ�����N2O4��ʾ�Ļ�ѧ��Ӧ���ʣ�
����NO2��ʾ�Ļ�ѧ��Ӧ���ʣ��ٸ�������֮�ȵ��ڼ�����֮�ȣ�����N2O4��ʾ�Ļ�ѧ��Ӧ���ʣ�
�ڸ�������ʽ���㷴Ӧ��N2O4�����ʵ�����ƽ��ʱ������������ʵ������ٸ���ƽ��ʱ��ϵ��ѹǿ�뿪ʼʱ��ϵ��ѹǿ֮��=ƽ��ʱ��������ʵ����뿪ʼʱ���ʵ���֮�ȼ��㣻
�۸��ݢڵļ���������N2O4��ƽ��ת���ʡ�
�ٿ�ʼ2 s����NO2��ʾ�Ļ�ѧ��Ӧ����v=![]() =0.04mol/(Ls)����������֮�ȵ��ڼ�����֮�ȣ���N2O4��ʾ�Ļ�ѧ��Ӧ����=
=0.04mol/(Ls)����������֮�ȵ��ڼ�����֮�ȣ���N2O4��ʾ�Ļ�ѧ��Ӧ����=![]() ��0.04mol/(Ls)= 0.02mol/(Ls)���ʴ�Ϊ��0.02mol/(Ls)��
��0.04mol/(Ls)= 0.02mol/(Ls)���ʴ�Ϊ��0.02mol/(Ls)��
���跴Ӧ��N2O4�����ʵ���Ϊx��
N2O4![]() 2NO2
2NO2
��ʼ(mol) 0.24 0
��Ӧ(mol) x 2x
ƽ��(mol) 0.24-x 2x
ƽ��ʱ������N2O4��NO2�����ʵ�����ȣ����0.24-x=2x�����x=0.08mol���ﵽƽ��ʱ��ϵ��ѹǿ�뿪ʼʱ��ϵ��ѹǿ֮��=ƽ��ʱ��������ʵ����뿪ʼʱ���ʵ���֮��=![]() =
=![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
�۸��ݢڵļ��㣬ƽ��ʱ��Ӧ��N2O4Ϊ0.08mol��N2O4��ƽ��ת����=![]() ��100%=33.3�����ʴ�Ϊ��33.3����
��100%=33.3�����ʴ�Ϊ��33.3����