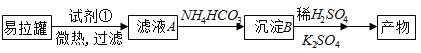��Ŀ����
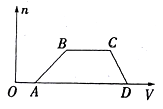
����Ŀ��ij�����Һ�п��ܺ���H2SO4��MgCl2��Al2(SO4)3��NH4Cl��NaCl�еļ������ʣ�������Һ������NaOH��Һ���������������ʵ���(n)������NaOH��Һ���(V)�Ĺ�ϵ��ͼ��ʾ���ش��������⣺
��1����Һ��һ�����е�������__________(�ѧʽ)��
��2����Һ��һ�������е�������__________(�ѧʽ)��
��3����Һ�п��ܺ��е�������__________(�ѧʽ)������ɫ��Ӧ�����ж��Ƿ��и����ʣ�������_________________��
��4���ֱ�д��AB�Ρ�CD�η��������ӷ���ʽ���� AB��Ϊ_________________����CD��Ϊ________________��
���𰸡���1��H2SO4��Al2(SO4)3��NH4Cl��
��2��MgCl2��
��3��NaCl������ʻ�ɫ��
��4��Al3++3OH-=Al(OH)3��; Al(OH)3+ OH-= AlO2-+ 2H2O��
��������
�������������ͼ���֪��OA��������˵����H2SO4��AB���г�����CD�γ�����ȫ��ʧ��˵������ΪAl(OH)3��˵����Һ��ֻ��Al2(SO4)3����MgCl2��BC�γ������䣬˵����Һ�к���NH4Cl���˶�笠����������������ӷ�Ӧ�����ݷ�����֪����Һ��һ������H2SO4��Al2(SO4)3��NH4Cl��һ������MgCl2�����ܺ���NaCl��
��1����Һ��һ������H2SO4��Al2(SO4)3��NH4Cl���ʴ�Ϊ��H2SO4��Al2(SO4)3��NH4Cl��
��2����Һ��һ������MgCl2���ʴ�Ϊ��MgCl2��
��3����Һ�п��ܴ��ڵ�����Ϊ�Ȼ���������ɫ��Ӧ�����ж��Ƿ��и����ʣ�����Ϊ����ʻ�ɫ���ʴ�Ϊ���Ȼ��أ�����ʻ�ɫ��
��4����AB�������������������ӷ�Ӧ��������������������Ӧ�����ӷ���ʽΪ��Al3++3OH-=Al(OH)3�����ʴ�Ϊ��Al3++3OH-=Al(OH)3����
��CD�����������������������ܽ�����Ӧ�����ӷ���ʽΪ��Al(OH)3+ OH-= AlO2-+ 2H2O���ʴ�Ϊ��Al(OH)3+ OH-= AlO2-+ 2H2O��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��Ԫ�ص��ʼ��仯�����й㷺��;����������ڱ��е�������Ԫ�����֪ʶ�ش��������⣺
��1����ԭ������������˳��ϡ��������⣩������˵����ȷ����________��
a��ԭ�Ӱ뾶�����Ӱ뾶����С
b�������Լ������ǽ�������ǿ
c���������Ӧ��ˮ������Լ�����������ǿ
d�����ʵ��۵㽵��
��2��ԭ�������������������������ͬ��Ԫ������Ϊ________�������������ļ���������________��
��3����֪��
������ | MgO | Al2O3 | MgCl2 | AlCl3 |
���� | ���ӻ����� | ���ӻ����� | ���ӻ����� | ���ۻ����� |
�۵�/�� | 2800 | 2050 | 714 | 191 |
��ҵ��þʱ�����MgCl2�������MgO��ԭ����_______________________________ ___��
����ʱ�����Al2O3�������AlCl3��ԭ����____________________________ __��
��4������裨�۵�1410 ���������õİ뵼����ϣ�SiCl4���۵�-70 �������ɴֹ��ƴ���������£�
![]()
SiCl4���� ���塣��������SiCl4�ƴ���ķ�Ӧ�У����ÿ����1.12 kg����������a kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��______________________________________ ��
��5��P2O5�Ƿ������Ը�������������岻����Ũ����������P2O5�������________��
a��NH3b��HI c��SO2d��CO2
����Ŀ��[��ѧ����ѡ��3�����ʽṹ������]
±��Ԫ�صĵ��ʺͻ�����ܶ࣬���ǿ���������ѧ���ʽṹ�����ʵ����֪ʶȥ��ʶ���������ǡ�
��1��±��Ԫ��λ�����ڱ���_________������ļ۵����Ų�ʽΪ_________ __________��
��2���ڲ�̫ϡ����Һ�У���������Զ����ӵ���HF��2��ʽ���ڵġ�ʹ�������ӵϵ���������________________��
��3��������±��ṩ�ĵ�һ�����������жϣ����п������ɽ��ȶ��ĵ��������ӵ�±��ԭ����_ _
�� | �� | �� | �� | �� | |
��һ������ ��kJ/mol�� | 1681 | 1251 | 1140 | 1008 | 900 |
��4������ˮ�е��ܽ����ȻС�����ڵ⻯����Һ���ܽ��ȴ������������������Һ�з������з�ӦI-+I2=I3-��I3-���ӵ�����ԭ����Χ�Ҽ����ӶԶ���Ϊ___ __���µ��ӶԶ���Ϊ__ ____
��KI3���Ƶģ�����CsICl2������֪CsICl2���ȶ��������ֽ⣬���������ɾ����ܸ�������ʣ�����������___ __ʽ������
A��CsICl2=CsCl+ICl
B��CsICl2=CsI+Cl2
��5����֪ClO2-ΪV�Σ�������ԭ����Χ���ĶԼ۲���ӡ�ClO2-������ԭ�ӵ��ӻ��������Ϊ___________��д��һ��ClO2-�ĵȵ�����__________��
��6����ͼΪ�⾧�徧���ṹ���й�˵������ȷ����__________��

A������ӵ�������2�ֲ�ͬ��ȡ����2��ȡ��ͬ
�ĵ��������λ��4������λ�γɲ�ṹ
B���þ�̯����֪ƽ��ÿ����������4����ԭ��
C���⾧��Ϊ��������Ŀռ�ṹ����ԭ�Ӿ���
D���⾧���еĵ�ԭ�Ӽ���ڷǼ��Լ��ͷ��»���
��7����֪CaF2��������ͼ�����ܶ�Ϊ��g/cm3��NAΪ�����ӵ����������ڵ�����Ca2+�ĺ˼��Ϊa cm����CaF2����Է����������Ա�ʾΪ__________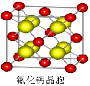 _��
_��