��Ŀ����
����Ŀ����������Ԫ�أ����� A��B��C��D Ϊ����������Ԫ�أ�E��F Ϊ��������Ԫ�أ����ǵ�ԭ��������������
A Ԫ��ԭ�ӵĺ��� p �������� s �������� 3 |
B Ԫ���γɵ���������࣬���γɵ�һ�ֹ��嵥�ʹ�ҵ�ϳ������и�� |
C Ԫ�ػ�̬ԭ�� p ����� 3 ��δ�ɶԵ��� |
D ԭ�Ӻ������� p ���ȫ������� |
E �ڸ�������δ�ɶԵ�������� |
F ���γɺ�ɫ����ש��ɫ���ͺ�ɫ������������ |
��������������Ϣ���ش����⣺
��1��A ��±�����ڹ�ҵ������Ҫ���ã�A ������±����ķе����±���ʾ��
±���� | AF3 | ACl3 | ABr3 | AI3 |
�е�/K | 172 | 285 | 364 | 483 |
������±����е��������ߵ�ԭ����_________________��
�� ACl3��LiAH4 ��A ԭ�ӵ��ӻ������������Ϊ______��_______���� A3N3H6 ��Ϊ�ȵ�����ķ��ӵĽṹ��ʽΪ___________��
����AF3���ӽṹ���ͷ�Ӧ AF3(g)+NH4F(s)=NH4AF4(s)�ܹ�������ԭ��_________________��
��2��ijͬѧ����������Ϣ���ƶ�
��B��̬ԭ�ӵĺ��������Ų�Ϊ ����ͬѧ�����ĵ����Ų�ͼΥ����________��
����ͬѧ�����ĵ����Ų�ͼΥ����________��
�� ��֪Ԫ�� B ��һ���⻯������Ҫ�Ļ���ԭ�ϣ����Ѹ��⻯��IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־���йظ��⻯����ӵ�˵����ȷ����________��
A�������к��з��Ӽ���� B�����ں��м��Լ��ķǼ��Է���
C��ֻ����4��sp-s�ĦҼ���1��p-p�Ħм� D�����⻯������� B ԭ�Ӳ��� sp2 �ӻ�
��3��D ��̬ԭ����������ߵĵ��ӣ���������ڿռ���______������ԭ�ӹ����______�Ρ�
��4��д�� E ԭ�ӵĵ����Ų�ʽ______________��
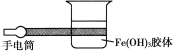
��5����д�� F Ԫ����Χ�����Ų�ʽ_________���� FSO4��Һ�еμ���CԪ���⻯���ˮ��Һ����������ɫ��������������ܽ⣬�õ�����ɫ����Һ����д�������ܽ�����ӷ���ʽ__________��
���𰸡��ṹ���ƣ������������Ӽ������������۷е��������� sp2�� sp3 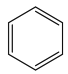 AF3 ��BF3 ��B���пչ������NH4F�е�F�йµ��Ӷԣ������ܹ���Ӧ ���ع��� BD 3 ���� 1s22s22p63s23p63d54s1 3d104s1 Cu(OH)2 +4NH3 = [Cu(NH3)4]2++2OH-
AF3 ��BF3 ��B���пչ������NH4F�е�F�йµ��Ӷԣ������ܹ���Ӧ ���ع��� BD 3 ���� 1s22s22p63s23p63d54s1 3d104s1 Cu(OH)2 +4NH3 = [Cu(NH3)4]2++2OH-
��������
��1��A��B��C��D Ϊ����������Ԫ�أ�E��F Ϊ��������Ԫ����A Ԫ��ԭ�ӵĺ��� p �������� s �������� 3�ĵ����Ų�Ϊ1S22S22P1֪AΪ��B��; B Ԫ���γɵ���������࣬���γɵ�һ�ֹ��嵥�ʹ�ҵ�ϳ������и��֪BΪ̼��C������C Ԫ�ػ�̬ԭ�� p ����� 3 ��δ�ɶԵ���֪����N����D ԭ�Ӻ������� p ���ȫ���������P����E �ڸ�������δ�ɶԵ����������Ϊ����Cr����F ���γɺ�ɫ����ש��ɫΪCuO���ͺ�ɫ(CuO)������������,����ΪCu;
����ΪAF3ΪBF3 BCl3 BBr3 BI3����±�����ǽṹ���ƣ��������������Ӽ������������۷е��������ߡ��𰸣��ṹ���ƣ������������Ӽ������������۷е������������� ACl3ΪBF3����ƽ�������Σ��ӻ���ʽΪsp2��LiAH4ΪLiBH4 ��B ԭ��Ϊsp3�����ӻ������������Ϊsp2 sp3���ȵ�����ָԭ��������ͬ���۵���������ͬ�ķ��ӣ��ȵ�����������ƵĻ�ѧ�������������� A3N3H6 ��Ϊ�ķ���ʽΪC6H6,�ṹ��ʽΪ![]() ��
��
��AF3�ķ���ʽΪBF3�� BF3 ��B���пչ������NH4F�е�F�йµ��Ӷԣ������ܹ���Ӧ��AF3(g)+NH4F(s)=NH4AF4(s)���𰸣�AF3 ��BF3 ��B���пչ������NH4F�е�F�йµ��Ӷԣ������ܹ���Ӧ��
��2�������ع���Ҫ������������ȵ���ռ��һ���������������״̬��ͬ��BΪ̼�����̬ԭ�ӵĺ��������Ų�ӦΪ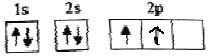 ������ͬѧ�����ĵ����Ų�ͼΥ���˺��ع��𰸣� ���ع�����
������ͬѧ�����ĵ����Ų�ͼΥ���˺��ع��𰸣� ���ع�����
��Ԫ��BΪ̼��һ���⻯������Ҫ�Ļ���ԭ��,���Ѹ��⻯��IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־,Ϊ��ϩ,�ṹʽΪ![]() ,����֪�������к���
,����֪�������к���![]() �Ǽ��Լ���
�Ǽ��Լ���![]() ���Լ�,Ϊƽ���η���,����5��
���Լ�,Ϊƽ���η���,����5��![]() ����1��
����1��![]() ��,ÿ��C�γ�3��
��,ÿ��C�γ�3��![]() ��,Ϊ
��,Ϊ![]() �ӻ�,��ˣ�������ȷ����:BD;
�ӻ�,��ˣ�������ȷ����:BD;
��3��DΪP���������Ų�Ϊ1S22S22P63S23P3, ��̬ԭ����������ߵĵ��ӣ���������ڿռ���3������ԭ�ӹ���������Ρ�
��4�� EΪ��Ϊ24��Ԫ�أ�����ԭ�ӵĵ����Ų�ʽ1s22s22p63s23p63d54s1��
��5�� F Ԫ��ΪCu,������Ų�ʽΪ1s22s22p63s23p63d104s1������Χ�����Ų�ʽ3d104s1 ��FSO4ΪCuSO4��Һ���� CuSO4��Һ�еμ���CԪ���⻯��ΪNH3,��ˮ��ҺΪ��ˮ����������ɫ����Cu(OH02����������ܽ⣬�õ�����ɫ����ҺΪ[Cu(NH3)4]2+���˳����ܽ�����ӷ���ʽCu(OH)2 +4NH3 = [Cu(NH3)4]2++2OH-��