��Ŀ����
����Ŀ��ij��ѧ��ȤС��ͬѧ�������������������Բ��˲�����֮˵������Ȥ�����ʵ��������̽�����������ױ����������е���Ԫ���Բ�����������ʽ���ڣ�����������һ�ֵ���ɫ������ˮ�ľ��塣
I.��������Ԫ�غ����IJⶨ
(1)ȡ100gϴ�����ɵ����ʲ��ˣ������װ��___________�У���������ճɻҽ���
(2)���ҽ���25mL 2mol/L������Һ�ܽ⣬�������һ���ӣ����˺�μ�����H2O2��Һ��ϡ����100mL��ȡ2mL����5��KSCN��Һ��H2O2�������ɹ�������Һ���ȱ�����ɫ����ɫ��ԭ����_________________________________��
(3)ȡ��ͬŨ�ȵ�___________[����(NH4)2Fe(SO4)2������NH4Fe(SO4)2��]����Һ��2mL���ֱ�μ�5��KSCN��Һ�������벽��(2)����Һ��ɫ��ӽ��ı���ҺŨ��Ϊ0.4��10-3mol/L��
(4)������ɵã�ÿ100g���ʲ����к�����Ԫ�ص�����ԼΪ___________ mg(����1λ��Ч����)��������5%�����ʣ�Ϊ����ÿ��20mg����Ԫ������ÿ����Ҫ�Բ���___________kg��
��.������������(FeC2O4��nH2O)�ķֽ�ʵ��
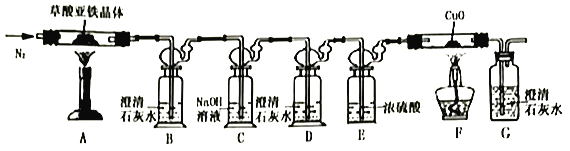
(1)�ӻ����Ƕȿ��ǣ�����ʵ��װ�õ�����ȱ����___________��
(2)ʵ�鿪ʼ��װ��B�г��ֻ���֤���ֽ�����д���CO��������___________������ַ�Ӧ��Ĺ��������Ͷ�뾭��е�ϡ�����У�������ȫ�ܽ���������ų���ȡ��ӦҺ����KSCN��Һ��Ѫ��ɫ��
(3)��ȡ7.2g���������������װ��AӲ�ʲ������У����Ⱥ�(��������跴Ӧ��ȫ)�����װ��AӲ�ʲ������в������2.88g��װ��FӲ�ʲ������й�����������0.64g���������������ֽ�Ļ�ѧ����ʽΪ_________________________________��
���𰸡����� H2O2��SCN-���� NH4Fe(SO4)2 2 20 ȱ��β������װ�� װ��D�в����ֻ��ǣ�װ��G�г��ֻ��ǣ���װ��F�ڹ����ɫ���ɫ FeC2O4��2H2O![]() FeO+CO��+CO2��+2H2O
FeO+CO��+CO2��+2H2O
��������
I.(1)������������Ҫ�������н��У�
(2)��H2O2���е������Է���;
(3)����Fe3+��SCN-��ΪѪ��ɫ������
II.(1)���������������ȷֽ�����һ����̼��һ����̼�ж�����ɢ�������л���Ⱦ������
(2)CO���л�ԭ�ԣ���F���ڼ���ʱ������Ӧ��CuO+CO![]() Cu+CO2��ͨ��D��ʯ��ˮ������ǣ�G��ʯ��ˮ������жϣ�
Cu+CO2��ͨ��D��ʯ��ˮ������ǣ�G��ʯ��ˮ������жϣ�
(3)����������FeO�����������ʵ����������������������ʵ�����F���ٵ�������OԪ�ص�����������CO��CuO��Ӧ�����ʵ�����ϵ�������CO�����ʵ���������CԪ���غ�ɵ�CO2�����ʵ������ٸ��������غ�ɵ�ˮ������������������ʵ����ıȵõ�������������Ļ�ѧʽ����ֽ�Ļ�ѧ����ʽ��
I.(1) �����ʵIJ���ϴ���������װ�������У���������ճɻҽ���
(2)��ϡ�����ܽ�ҽ����������һ���ӣ�ʹ��Ӧ���ֽӴ����ܽ⣬���˳�ȥ����������Һ�еμ�����H2O2��Һ������Һ�е�Fe2+����ΪFe3+������KSCN��Һ����Fe3+�Ĵ��ڣ���H2O2������������H2O2����ǿ�������ԣ��Ὣ��Һ��SCN-����Ϊ(SCN)2���壬ʹ��Һ�ȱ�����ɫ��
(3)�ڲ���(2)����Ԫ�ر�Ϊ+3�۵�Fe3+��Fe3+��KSCN��Һ��Ϊ��ɫ����(NH4)2Fe(SO4)2��FeΪ+2�ۣ���NH4Fe(SO4)2��FeΪ+3�ۣ�����ѡ��ı���ҺΪNH4Fe(SO4)2��100g���˵Ļҽ����Ƴ���100mL��Һ������2mL��Һ�к���Fe�����ʵ���Ϊn=0.4��10-3mol/L��0.002L=8��10-6mol����100g���ʲ����к�����Ԫ�ص�����m(Fe)= 8��10-6mol��![]() =2.24��10-3g=2.24mg��2mg��������5%�����ʣ�Ϊ����ÿ��20mg����Ԫ������ÿ��һ������Fe������Ϊm=20mg��5%=400mg=0.4g������100g���˺���Fe����Ϊ2mg������һ����һ����Ҫ�Բ���(400mg��100)��2=20000g=20kg��
=2.24��10-3g=2.24mg��2mg��������5%�����ʣ�Ϊ����ÿ��20mg����Ԫ������ÿ��һ������Fe������Ϊm=20mg��5%=400mg=0.4g������100g���˺���Fe����Ϊ2mg������һ����һ����Ҫ�Բ���(400mg��100)��2=20000g=20kg��
��.(1)���������������ȷֽ�����FeO��CO��CO2��H2O��һ����̼���ж����壬��ɢ�������л���Ⱦ���������Դӻ����Ƕȿ��ǣ�����ʵ��װ�õ�����ȱ����û�д���β��������
(2)��װ��A�в��������������ȷֽ⣬��Ӧ����ʽ��FeC2O4��2H2O![]() FeO+CO��+CO2��+2H2O������������ͨ��װ��B������CO2���壬Ȼ��ͨ��װ��C���ճ�ȥCO2��Ȼ��ͨ��װ��D����CO2�Ѿ������ɾ�����ͨ��E�е�Ũ�������CO����ĸ����F��CO��CuO�ڼ���ʱ������Ӧ��CuO+CO
FeO+CO��+CO2��+2H2O������������ͨ��װ��B������CO2���壬Ȼ��ͨ��װ��C���ճ�ȥCO2��Ȼ��ͨ��װ��D����CO2�Ѿ������ɾ�����ͨ��E�е�Ũ�������CO����ĸ����F��CO��CuO�ڼ���ʱ������Ӧ��CuO+CO![]() Cu+CO2��������CO2�������Gװ�ã��ɿ�������ʯ��ˮ����ǣ���װ��F�ڹ����ɫ���ɫ���Ӷ���֤����Ӧ������CO���壻
Cu+CO2��������CO2�������Gװ�ã��ɿ�������ʯ��ˮ����ǣ���װ��F�ڹ����ɫ���ɫ���Ӷ���֤����Ӧ������CO���壻
(3)n(FeO)=2.88g��72g/mol=0.04mol������FeԪ���غ��֪����������������ʵ�����0.04mol�����ݷ���ʽCuO+CO![]() Cu+CO2��֪F�м��ٵ�������CuO��OԪ�ص������������ʵ�����CO�����ʵ�����ȣ���n(CO)=n(O)=
Cu+CO2��֪F�м��ٵ�������CuO��OԪ�ص������������ʵ�����CO�����ʵ�����ȣ���n(CO)=n(O)=![]() �����ڲ�����������ֽ�ʱ����CO��CO2�����ʵ�����ȣ�����n(CO2)= n(CO)=0.04mol��m(CO)=0.04mol��28g/mol=1.12g��m(CO2)= 0.04mol��44g/mol=1.76g�����Ը��ݻ�ѧ��Ӧ�����������غ㣬��֪��Ӧ������ˮ��������m(H2O)= 7.2g-2.88g-1.12g-1.76g=1.44g��n(H2O)=1.44g��18g/mol=0.08mol������n(����)��n(FeO)��n(CO)��n(CO2)��n(H2O)=0.04��0.04��0.04��0.04��0.08=1��1��1��1��2�����Բ����������廯ѧʽ��FeC2O4��2H2O�������ʷֽ�Ļ�ѧ����ʽΪ��
�����ڲ�����������ֽ�ʱ����CO��CO2�����ʵ�����ȣ�����n(CO2)= n(CO)=0.04mol��m(CO)=0.04mol��28g/mol=1.12g��m(CO2)= 0.04mol��44g/mol=1.76g�����Ը��ݻ�ѧ��Ӧ�����������غ㣬��֪��Ӧ������ˮ��������m(H2O)= 7.2g-2.88g-1.12g-1.76g=1.44g��n(H2O)=1.44g��18g/mol=0.08mol������n(����)��n(FeO)��n(CO)��n(CO2)��n(H2O)=0.04��0.04��0.04��0.04��0.08=1��1��1��1��2�����Բ����������廯ѧʽ��FeC2O4��2H2O�������ʷֽ�Ļ�ѧ����ʽΪ��![]() FeO+CO��+CO2��+2H2O��
FeO+CO��+CO2��+2H2O��

����Ŀ����������Ԫ�أ����� A��B��C��D Ϊ����������Ԫ�أ�E��F Ϊ��������Ԫ�أ����ǵ�ԭ��������������
A Ԫ��ԭ�ӵĺ��� p �������� s �������� 3 |
B Ԫ���γɵ���������࣬���γɵ�һ�ֹ��嵥�ʹ�ҵ�ϳ������и�� |
C Ԫ�ػ�̬ԭ�� p ����� 3 ��δ�ɶԵ��� |
D ԭ�Ӻ������� p ���ȫ������� |
E �ڸ�������δ�ɶԵ�������� |
F ���γɺ�ɫ����ש��ɫ���ͺ�ɫ������������ |
��������������Ϣ���ش����⣺
��1��A ��±�����ڹ�ҵ������Ҫ���ã�A ������±����ķе����±���ʾ��
±���� | AF3 | ACl3 | ABr3 | AI3 |
�е�/K | 172 | 285 | 364 | 483 |
������±����е��������ߵ�ԭ����_________________��
�� ACl3��LiAH4 ��A ԭ�ӵ��ӻ������������Ϊ______��_______���� A3N3H6 ��Ϊ�ȵ�����ķ��ӵĽṹ��ʽΪ___________��
����AF3���ӽṹ���ͷ�Ӧ AF3(g)+NH4F(s)=NH4AF4(s)�ܹ�������ԭ��_________________��
��2��ijͬѧ����������Ϣ���ƶ�
��B��̬ԭ�ӵĺ��������Ų�Ϊ ����ͬѧ�����ĵ����Ų�ͼΥ����________��
����ͬѧ�����ĵ����Ų�ͼΥ����________��
�� ��֪Ԫ�� B ��һ���⻯������Ҫ�Ļ���ԭ�ϣ����Ѹ��⻯��IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־���йظ��⻯����ӵ�˵����ȷ����________��
A�������к��з��Ӽ���� B�����ں��м��Լ��ķǼ��Է���
C��ֻ����4��sp-s�ĦҼ���1��p-p�Ħм� D�����⻯������� B ԭ�Ӳ��� sp2 �ӻ�
��3��D ��̬ԭ����������ߵĵ��ӣ���������ڿռ���______������ԭ�ӹ����______�Ρ�
��4��д�� E ԭ�ӵĵ����Ų�ʽ______________��
��5����д�� F Ԫ����Χ�����Ų�ʽ_________���� FSO4��Һ�еμ���CԪ���⻯���ˮ��Һ����������ɫ��������������ܽ⣬�õ�����ɫ����Һ����д�������ܽ�����ӷ���ʽ__________��
