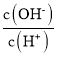��Ŀ����
����Ŀ����ϩ����Ҫ�Ĺ�ҵƷ����������ȡ±���������������ϵȡ��ش��������⣺
(1)��ϩ��HCl�ӳ�����CH3CHClCH3��CH3CH2CH2Cl�������뷴Ӧ������ͼ��
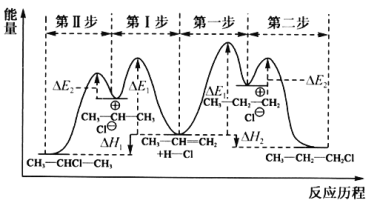
���������Ƕȿ������ȶ��IJ�����________(����CH3CHClCH3������CH3CH2CH2Cl��)��
����ȡCH3CHClCH3ʱ����Ӧ���ʽ����IJ�����________(������������)��
(2)����ֱ�������Ʊ�ϩ������Ȼ�ѧ��Ӧ���£�
����Ӧ��C3H8(g)��C3H6(g)+H2 ��H1=+124.27kJ��mol-1
����Ӧ��C3H8(g)��CH4(g)+C2H4(g) ��H2=+81.30kJ��mol-1
C3H8(g)+H2(g)��CH4(g)+C2H6(g) ��H3=-55.64kJ��mol-1
C2H4(g)+H2(g)��C2H6(g) ��H4
�٦�H4=_____________kJ��mol-1
���ֱ���0.1Mpa��0.01Mpaʱ������ͱ�ϩ��ƽ�����ʵ���������ͼ��ʾ��
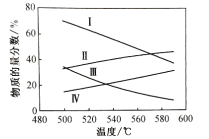
��ʾ0.1Mpaʱ�������ʵ�����������________(���������֣���ͬ)����ʾ0.01Mpaʱ��ϩ�������ķ�������________��
(3)�ö�����̼���������Ʊ�ϩ��Ҫ��Ӧ���£�
����C3H8(g)![]() C3H6(g)+ H2 ��H1>0ƽ�ⳣ��K1
C3H6(g)+ H2 ��H1>0ƽ�ⳣ��K1
����CO2(g)+H2(g)![]() CO(g)+H2O(g)����H2>0ƽ�ⳣ��K2
CO(g)+H2O(g)����H2>0ƽ�ⳣ��K2
������Ϸ�ӦC3H8(g)+CO2(g)![]() C3H6(g)+CO(g)+H2O(g)
C3H6(g)+CO(g)+H2O(g)
��C3H8�����ʵ���һ��ʱ������ͬͶ�ϱ�Z[Z=![]() ]���ܱ������г���C3H8��CO2��������Ӧ����C3H8ƽ��ת�������¶ȱ仯��ͼ��ʾ��
]���ܱ������г���C3H8��CO2��������Ӧ����C3H8ƽ��ת�������¶ȱ仯��ͼ��ʾ��
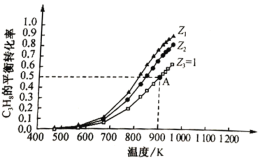
����Ӧ����ƽ�ⳣ��K=________(��K1��K2��ʾ)��
��Z1________(����>����<����=��)Z2��ԭ����________
����A��������ѹǿΪ0.2Mpa�����ϩ��ƽ���ѹΪ________MPa��900Kʱ��Ӧ����ƽ�ⳣ��Kp=________MPa(KpΪ��ƽ���ѹ��ʾ��ƽ�ⳣ��)��
���𰸡�CH3CHClCH3 I -136.94 I II K1K2 > ZԽ��c(CO2)Խ��C3H8��ƽ��ת����Խ�� 0.04 0.04
��������
(1)��һ������£���������Խ�ͣ� Խ�ȶ�����ͼ�п�֪��CH3CHClCH3���е���������CH3CH2CH2Cl����˽��ȶ��IJ�����CH3CHClCH3��
��һ������£���Ӧ�Ļ��Խ��ѧ��Ӧ����Խ������ͼʾ���Կ�����CH3CH=CH2����CH3CHClCH3�Ĺ����У��������Ļ�ܴ�Ӧ���ʽ�����
(2)�ٸ��ݸ�˹���ɣ�����Ӧ��C3H8(g)��CH4(g)+C2H4(g) ��H2=+81.30kJ��mol-1����C3H8(g)+H2(g)��CH4(g)+C2H6(g) ��H3=-55.64kJ��mol-1����Ӧ�ڣ��ٿɵ�Ŀ�귴ӦC2H4(g)+H2(g)��C2H6(g)������H4=��H3-��H2=-55.64kJ��mol-1-(+81.30kJ��mol-1)= -136.94kJ��mol-1��
������ӦΪ���ȷ�Ӧ����������һ���£������¶ȣ�ƽ�������ƶ�����������ʵ���������С������ϩ�����ʵ����������ӣ�����ӦΪ�����������ķ�Ӧ����������һ���£�����ѹǿ��ƽ�������ƶ�����������ʵ�����������ϩ�����ʵ���������С����֪������������ʾ��������ʵķ����ı仯��ѹǿԽ��������ʵ�������Խ����˱�ʾ0.1MPaʱ���������ʵ�������������������������������ϩ�����ʵ��������ı仯��ѹǿԽ��ϩ�����ʵ�������ԽС�����ʾ0.01Mpaʱ��ϩ�������ķ�����������
(3)�ٸ��ݸ�˹���ɣ���Ӧ������Ӧ���ɵ÷�Ӧ������K3=K1K2��
������ͬ�¶��£�c(CO2)Խ��C3H8��ת����Խ��֪Z1>Z2����ΪZԽ��c(CO2)Խ��C3H8��ת����Խ��
��A��������ѹǿΪ0.2Mpa��C3H8��ת����Ϊ0.5��Z=1������n(CO2)=n(C3H8)=1mol����������ʽ����
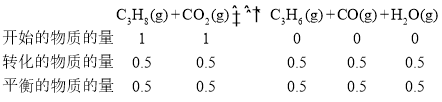
C3H8��ƽ���ѹp(C3H8)=![]() ������ƽ��ʱ�������ʵ����ʵ�����ͬ����˸����ʵķ�ѹ��ͬ����900���£���Ӧ���ƽ�ⳣ��
������ƽ��ʱ�������ʵ����ʵ�����ͬ����˸����ʵķ�ѹ��ͬ����900���£���Ӧ���ƽ�ⳣ��![]() ��
��