题目内容
【题目】H2S与CO2高温下发生反应:H2S(g)+CO2(g) ![]() COS(g)+H2O(g)。在610 K时,将0.10 mol CO2与0.40 molH2S充入2.5 L的空钢瓶中,反应平衡后水的物质的量分数为0.02。
COS(g)+H2O(g)。在610 K时,将0.10 mol CO2与0.40 molH2S充入2.5 L的空钢瓶中,反应平衡后水的物质的量分数为0.02。
(1)H2S的平衡转化率α1=________%,反应平衡常数K=___________________。
(2)在620K重复实验,平衡后水的物质的量分数为0.03,H2S的转化率α2________α1,该反应的ΔH____0(填“>”“<”或“=”)。
(3)向反应器中再分别充入下列气体,能使H2S转化率增大的是______(填标号)。
A.H2S B.CO2 C.COS D.N2
【答案】2.52.85×10-3(或![]() )>>B
)>>B
【解析】
列“三段式”解题
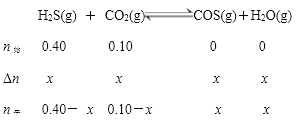
水的物质的量分数为![]() =0.02
=0.02
解得x=0.01
(1)α1=![]() ×100%=2.5%,K=
×100%=2.5%,K= =
=![]() ,故答案为: 2.5、2.85×10-3
,故答案为: 2.5、2.85×10-3![]() ;(2)升温,水的物质的量分数升高,说明升温时平衡正移,则α2>α1,ΔH>0,故答案为:>、>;(3)A.通入H2S,平衡虽正向移动,但H2S的转化率减小,故A项错误;B.通入CO2,平衡正向移动,H2S转化率增大,故B正确;C.通入COS,增大生成物浓度,平衡逆向移动,H2S转化率减小,故C错误;D.通入N2,平衡不移动,H2S转化率不变,故D错误;答案选B。
;(2)升温,水的物质的量分数升高,说明升温时平衡正移,则α2>α1,ΔH>0,故答案为:>、>;(3)A.通入H2S,平衡虽正向移动,但H2S的转化率减小,故A项错误;B.通入CO2,平衡正向移动,H2S转化率增大,故B正确;C.通入COS,增大生成物浓度,平衡逆向移动,H2S转化率减小,故C错误;D.通入N2,平衡不移动,H2S转化率不变,故D错误;答案选B。

 天天向上一本好卷系列答案
天天向上一本好卷系列答案 小学生10分钟应用题系列答案
小学生10分钟应用题系列答案【题目】亚硝酰氯(NOCl,熔点:-64.5 ℃,沸点:-5.5 ℃)是一种黄色气体,遇水易反应生成一种无氧酸和两种氮的常见氧化物。亚硝酰氯应用广泛,可用于合成清洁剂、触媒剂及中间体等。实验室可由氯气与一氧化氮在常温常压下合成。
(1)甲组同学拟制备原料气NO和Cl2,制备装置如图所示,为制备纯净干燥的气体,补充右表中缺少的药品。
装置Ⅰ | 装置Ⅱ | ||
蒸馏烧瓶中 | A仪器中 | ||
制备纯净的Cl2 | MnO2 | ①______ | ②______ |
制备纯净的NO | Cu | ③______ | ④______ |
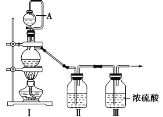
(2)乙组同学对甲组同学制取NO的装置略加改良,结合甲组制得的Cl2共同制备NOCl,装置如图所示:
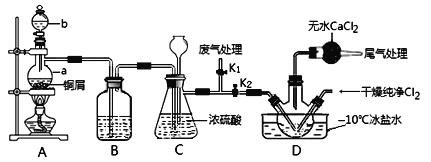
①仪器b的名称为________________。
②组装好实验装置后应先______________,然后依次装入药品。此实验关键操作有两点:一是将Cl2充满D装置的三颈瓶中;二是A中反应开始时要先关闭K2,打开K1,待NO充满装置后再关闭K1,打开K2。这两步操作中充满的目的都是__________________________________________________。
③若C装置中压强过大,可以观察到的现象是_________________________。
④装置D中冰盐水的作用是__________________。
(3)亚硝酰氯(NOCl)纯度的测定:将所得亚硝酰氯(NOCl)产品13.10g溶于水,配制成250mL溶液;取出25.00mL,以K2CrO4溶液为指示剂,用0.8mol·L-1AgNO3标准溶液滴定至终点,消耗标准溶液的体积为 22.50mL。(已知:Ag2CrO4为砖红色固体)
①亚硝酰氯(NOCl)与水反应的化学方程式为_________________________。
②亚硝酰氯(NOCl)的质量分数为______________________。